From Wikipedia, the free encyclopedia
Semicarbazide
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.000.308
KEGG
InChI=1S/CH5N3O/c2-1(5)4-3/h3H2,(H3,2,4,5)
Y Key: DUIOPKIIICUYRZ-UHFFFAOYSA-N
Y InChI=1/CH5N3O/c2-1(5)4-3/h3H2,(H3,2,4,5)
Key: DUIOPKIIICUYRZ-UHFFFAOYAJ
Properties
H2 NNHC(=O)NH2
Molar mass
75.08 g/mol
Melting point
96 °C
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
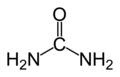
Semicarbazide is the chemical compound with the formula OC(NH2 )(N2 H3 ). This water-soluble white solid is also known as carbohydrazide. It is a derivative of urea.
Synthesis
The compound prepared by treating urea with hydrazine :[ 1]
OC(NH2 )2 + N2 H4 → OC(NH2 )(N2 H3 ) + NH3 A further reaction can occur to give carbohydrazide:
OC(NH2 )(N2 H3 ) + N2 H4 → OC(N2 H3 )2 + NH3
Derivatives
A thiosemicarbazide is the analog with sulfur atom in place of oxygen atom, with 4-Methyl-3-thiosemicarbazide being a simple example. Semicarbazones are derived by the condensation reaction between a ketone (or aldehyde ) and a semicarbazide.
Properties
Semicarbazide products (semicarbazones and thiosemicarbazones) are known to have an activity of antiviral , antiinfective and antineoplastic through binding to copper or iron in cells.
Uses
Semicarbazide is used in preparing pharmaceuticals including nitrofuran antibacterials (furazolidone , nitrofurazone , nitrofurantoin ) and related compounds. Semicarbazide is used as a detection reagent in thin layer chromatography (TLC). Semicarbazide stains α-keto acids on the TLC plate, which must then be viewed under ultraviolet light to see the results.
Occurrence
Semicarbazide has been shown to be formed in heat-treated flour containing azodicarbonamide as well as breads made from azodicarbonamide-treated flour.[ 2]
Structures
See also
References
^ Jean-Pierre Schirmann, Paul Bourdauducq "Hydrazine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. doi :10.1002/14356007.a13_177 .
^ Becalski, Adam; Lau, Benjamin; Lewis, David; Seaman, Stephen (2004). "Semicarbazide Formation in Azodicarbonamide-Treated Flour: A Model Study". J. Agric. Food Chem . 52 (18): 5730.
External links
Ionotropic
GABAA Tooltip γ-Aminobutyric acid A receptor
Positive modulators (abridged; see here for a full list): α-EMTBL Alcohols (e.g., drinking alcohol , 2M2B )Anabolic steroids Avermectins (e.g., ivermectin )Barbiturates (e.g., phenobarbital )Benzodiazepines (e.g., diazepam )Bromide compounds (e.g., potassium bromide )Carbamates (e.g., meprobamate )Carbamazepine Chloralose Chlormezanone Clomethiazole Dihydroergolines (e.g., ergoloid (dihydroergotoxine) )Etazepine Etifoxine Fenamates (e.g., mefenamic acid )Flavonoids (e.g., apigenin , hispidulin )Fluoxetine Flupirtine Imidazoles (e.g., etomidate )Kava constituents (e.g., kavain )Lanthanum Loreclezole Monastrol Neuroactive steroids (e.g., allopregnanolone , cholesterol , THDOC )Niacin Niacinamide Nonbenzodiazepines (e.g., β-carbolines (e.g., abecarnil ), cyclopyrrolones (e.g., zopiclone ), imidazopyridines (e.g., zolpidem ), pyrazolopyrimidines (e.g., zaleplon ))Norfluoxetine Petrichloral Phenols (e.g., propofol )Phenytoin Piperidinediones (e.g., glutethimide )Propanidid Pyrazolopyridines (e.g., etazolate )Quinazolinones (e.g., methaqualone )Retigabine (ezogabine) ROD-188 Skullcap constituents (e.g., baicalin )Stiripentol Sulfonylalkanes (e.g., sulfonmethane (sulfonal) )Topiramate Valerian constituents (e.g., valerenic acid )Volatiles /gases (e.g., chloral hydrate , chloroform , diethyl ether , paraldehyde , sevoflurane )Negative modulators: 1,3M1B 3M2B 11-Ketoprogesterone 17-Phenylandrostenol α3IA α5IA (LS-193,268) β-CCB β-CCE β-CCM β-CCP β-EMGBL Anabolic steroids Amiloride Anisatin β-Lactams (e.g., penicillins , cephalosporins , carbapenems )Basmisanil Bemegride Bicyclic phosphates (TBPS , TBPO , IPTBO )BIDN Bilobalide Bupropion CHEB Chlorophenylsilatrane Cicutoxin Cloflubicyne Cyclothiazide DHEA DHEA-S Dieldrin (+)-DMBB DMCM DMPC EBOB Etbicyphat FG-7142 (ZK-31906) Fiproles (e.g., fipronil )Flavonoids (e.g., amentoflavone , oroxylin A )Flumazenil Fluoroquinolones (e.g., ciprofloxacin )Flurothyl Furosemide Golexanolone Iomazenil (123 I) IPTBO Isopregnanolone (sepranolone) L-655,708 Laudanosine Lindane MaxiPost Morphine Morphine-3-glucuronide MRK-016 Naloxone Naltrexone Nicardipine Nonsteroidal antiandrogens (e.g., apalutamide , bicalutamide , enzalutamide , flutamide , nilutamide )Oenanthotoxin Pentylenetetrazol (pentetrazol) Phenylsilatrane Picrotoxin (i.e., picrotin , picrotoxinin and dihydropicrotoxinin )Pregnenolone sulfate Propybicyphat PWZ-029 Radequinil Ro 15-4513 Ro 19-4603 RO4882224 RO4938581 Sarmazenil SCS Suritozole TB-21007 TBOB TBPS TCS-1105 Terbequinil TETS Thujone U-93631 Zinc ZK-93426 GABAA -ρ Tooltip γ-Aminobutyric acid A-rho receptor
Metabotropic
GABAB Tooltip γ-Aminobutyric acid B receptor