Lithium iodate: Difference between revisions
Appearance
Content deleted Content added
Dr. Blofeld (talk | contribs) No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Chembox |
|||
| ⚫ | '''Lithium |
||
| verifiedrevid = 464390892 |
|||
| ImageFile = LiIO3-skel.svg |
|||
| ImageSize = 120px |
|||
| ImageName = Skeletal formula of lithium iodate with I—O bond length |
|||
| ImageFile1 = LiIO3-polyhedral.png |
|||
| ImageSize1 = 160px |
|||
| ImageName1 = Crystal structure of lithium iodate, iodines are inside the unit cell |
|||
| IUPACName = Lithium iodate |
|||
| Section1 = {{Chembox Identifiers |
|||
| CASNo = 13765-03-2 |
|||
| CASNo_Ref = {{cascite|correct|CAS}} |
|||
| PubChem = 3084149 |
|||
| PubChem_Ref = {{pubchemcite}} |
|||
| ChemSpiderID = 141432 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| EC-number = 237-365-2 |
|||
| SMILES = [Li+].[O-]I(=O)=O |
|||
| SMILES_Ref = {{smilescite}} |
|||
| StdInChI = 1S/HIO3.Li/c2-1(3)4;/h(H,2,3,4);/q;+1/p-1 |
|||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = FZAXZVHFYFGNBX-UHFFFAOYSA-M |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| UNNumber = 1479 |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| Li=1|I=1|O=3 |
|||
| Appearance = White [[hygroscopic]] crystals |
|||
| Odor = Odorless |
|||
| Density = 4.487 g/cm<sup>3</sup><ref name=webc /> |
|||
| MeltingPtC = 420-450 |
|||
| Melting_ref = <ref name=webc /><ref name=crc>{{CRC90}}</ref><ref name=spie /> |
|||
| Solubility = Anhydrous:<br> 89.4 g/100 mL (10 °C)<br> 82.7 g/100 mL (25 °C)<br> 78.4 g/100 mL (40.1 °C)<br> 73 g/100 mL (75.6 °C)<ref name=webc /><br> Hemihydrate:<br> 80.2 g/100 mL (18 °C)<ref name=sioc>{{cite book|last = Seidell|first = Atherton|last2 = Linke|first2 = William F.|year = 1919|title = Solubilities of Inorganic and Organic Compounds|publisher = D. Van Nostrand Company|place = [[New York]]|edition = 2nd|page = 374}}</ref> |
|||
| SolubleOther = Insoluble in [[ethanol|EtOH]]<ref name=crc /> |
|||
| RefractIndex = 1.8875 (20 °C)<br> 1.6 (RT)<br> ''n''<sub>[[Helium–neon laser|He–Ne]]</sub>:<br> 1.8815 (20 °C)<ref name=webc /><br> 1.5928 (RT)<ref>{{cite web|url = http://refractiveindex.info/?shelf=main&book=LiIO3&page=Herbst-o|title = Refractive index of LiIO3 (Lithium iodate) - Herbst-o|website = http://www.refractiveindex.info|first = Mikhail|last = Polyanskiy|accessdate = 2014-08-08}}</ref> |
|||
| ThermalConductivity = 1.27 W/m·K (a-axis)<br> 0.65 W/m·K (c-axis)<ref name=webc /> |
|||
}} |
|||
| Section3 = {{Chembox Structure |
|||
| CrystalStruct = [[Hexagonal crystal system|Hexagonal]],<ref name=crc /> [[Pearson symbol|hP10]]<ref name=aps>{{cite journal|title = Crystal Structure of Lithium Iodate|first1 = W.H.|last1 = Zachariasen|first2 = F.A. BartaLars|last2 = Olof|journal = [[Physical Review Letters]]|date = 1931-06-15|volume = 37|pages = 1626|doi = 10.1103/PhysRev.37.1626}}</ref> |
|||
| SpaceGroup = P6<sub>3</sub>22, No. 182<ref name=aps /> |
|||
| PointGroup = 622<ref name=aps /> |
|||
| LattConst_a = 5.46(9) Å |
|||
| LattConst_c = 5.15(5) Å<ref name=aps /> |
|||
| LattConst_gamma = 120 |
|||
}} |
|||
| Section7 = {{Chembox Hazards |
|||
| GHSPictograms = {{GHS03}}{{GHS07}}{{GHS08}}<ref name="sigma">{{Sigma-Aldrich|id=443964|name=Lithium iodate|accessdate=2014-08-08}}</ref> |
|||
| GHSSignalWord = Danger |
|||
| HPhrases = {{H-phrases|272|315|319|335|360}}<ref name="sigma" /> |
|||
| PPhrases = {{P-phrases|201|220|261|305+351+338|308+313}}<ref name="sigma" /> |
|||
| RPhrases = {{R8}}, {{R36/37/38}}, {{R61}} |
|||
| SPhrases = {{S17}}, {{S22}}, {{S36/37/39}}, {{S45}}, {{S53}} |
|||
| EUClass = {{Hazchem O}} {{Hazchem T}} |
|||
| NFPA-H = 2 |
|||
| NFPA-F = 0 |
|||
| NFPA-R = 2 |
|||
| NFPA_Ref = <ref name=pab>{{cite web|url = https://www.pfaltzandbauer.com/MSDS/L04180%20%20SDS%20%2005202013.pdf|publisher = Pfaltz & Bauer, Inc.|place = [[Connecticut]], USA|accessdate = 2014-08-08|website = https://www.pfaltzandbauer.com|title = SDS of Lithium iodate anhydrous}}</ref> |
|||
}} |
|||
}} |
|||
| ⚫ | '''Lithium iodate''' (LiIO<sub>3</sub>) is a negative uniaxial crystal<ref name=webc>{{cite book|title = Rarely Used and Archive Crystals|journal = Nonlinear Optical Crystals: A Complete Survey|year = 2005|doi = 10.1007/0-387-27151-1_8|isbn = 978-0-387-27151-4|pages = 364–368|url = http://www.webcryst.com/zh/download/finish/6-/477-liio3-lithium-iodate.html}}</ref> for nonlinear, acousto-optical and piezoelectric applications. It has been utilized for 347 nm ruby lasers.<ref name="RiskGosnell2003">{{cite book|last1=Risk|first1=W. P.|last2=Gosnell|first2=T. R.|last3=Nurmikko|first3=A. V.|title=Compact Blue-Green Lasers|url=http://books.google.com/books?id=rtLwj5H9JacC&pg=PA123|accessdate=13 December 2012|date=9 January 2003|publisher=[[Cambridge University Press]]|isbn=978-0-521-52103-1|page=123}}</ref><ref name="Nikogosyan2005">{{cite book|last=Nikogosyan|first=David N.|title=Nonlinear Optical Crystals: A Complete Survey|url=http://books.google.com/books?id=ZW9Ynx_Z7kkC&pg=PA371|accessdate=13 December 2012|date=4 January 2005|publisher=Springer|isbn=978-0-387-22022-2|page=371}}</ref> |
||
==Properties== |
|||
[[Mohs hardness]] of lithium iodate is 3.5–4. Its linear [[thermal expansion]] coefficient at {{convert|298|K|C F}} is 2.8·10<sup>−5</sup>/°C (a-axis) and 4.8·10<sup>−5</sup>/°C (c-axis).<ref name=webc /> Its transition to β-form begin at {{convert|50|C|F}} and it is irreversible.<ref name=spie>{{cite journal|title = LiIO<sub>3</sub> nanocrystals in SiO<sub>2</sub> xerogels, a new material for non-linear optics|url = http://www.researchgate.net/profile/Yannick_Mugnier/publication/252139042_LiIO3_nanocrystals_in_SiO2_xerogels_a_new_material_for_nonlinear_optics/links/00b4952a1772abcc77000000|journal = Proceeding SPIE|volume = 5222|issue = 26|date = 2003-11-20|first1 = Jeremie|last1 = Teyssier|first2 = Ronan Le|last2 = Dantec|first3 = Christine|last3 = Galez|first4 = Yannick|last4 = Mugnier|first5 = Jacques|last5 = Bouillot|first6= Jean-Claude|last6 = Plenet|doi = 10.1117/12.507309}}</ref> |
|||
==References== |
==References== |
||
{{reflist}} |
{{reflist}} |
||
{{Lithium compounds}} |
|||
{{Inorganic-compound-stub}} |
|||
[[Category:Lithium minerals]] |
[[Category:Lithium minerals]] |
||
Revision as of 17:04, 8 August 2014
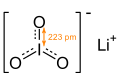
| |

| |
| Names | |
|---|---|
| IUPAC name
Lithium iodate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.954 |
PubChem CID
|
|
| UN number | 1479 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ILiO3 | |
| Molar mass | 181.84 g·mol−1 |
| Appearance | White hygroscopic crystals |
| Odor | Odorless |
| Density | 4.487 g/cm3[1] |
| Melting point | 420–450 °C (788–842 °F; 693–723 K) |
| Anhydrous: 89.4 g/100 mL (10 °C) 82.7 g/100 mL (25 °C) 78.4 g/100 mL (40.1 °C) 73 g/100 mL (75.6 °C)[1] Hemihydrate: 80.2 g/100 mL (18 °C)[2] | |
| Solubility | Insoluble in EtOH[3] |
| Thermal conductivity | 1.27 W/m·K (a-axis) 0.65 W/m·K (c-axis)[1] |
Refractive index (nD)
|
1.8875 (20 °C) 1.6 (RT) nHe–Ne: 1.8815 (20 °C)[1] 1.5928 (RT)[4] |
| Structure | |
| Hexagonal,[3] hP10[6] | |
| P6322, No. 182[6] | |
| 622[6] | |
a = 5.46(9) Å, c = 5.15(5) Å[6] α = 90°, β = 90°, γ = 120°
| |
| Hazards | |
| GHS labelling: | |
   [7] [7]
| |
| Danger | |
| H272, H315, H319, H335, H360[7] | |
| P201, P220, P261, P305+P351+P338, P308+P313[7] | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Lithium iodate (LiIO3) is a negative uniaxial crystal[1] for nonlinear, acousto-optical and piezoelectric applications. It has been utilized for 347 nm ruby lasers.[9][10]
Properties
Mohs hardness of lithium iodate is 3.5–4. Its linear thermal expansion coefficient at 298 K (25 °C; 77 °F) is 2.8·10−5/°C (a-axis) and 4.8·10−5/°C (c-axis).[1] Its transition to β-form begin at 50 °C (122 °F) and it is irreversible.[5]
References
- ^ a b c d e f g Rarely Used and Archive Crystals. 2005. pp. 364–368. doi:10.1007/0-387-27151-1_8. ISBN 978-0-387-27151-4.
{{cite book}}:|journal=ignored (help) - ^ Seidell, Atherton; Linke, William F. (1919). Solubilities of Inorganic and Organic Compounds (2nd ed.). New York: D. Van Nostrand Company. p. 374.
- ^ a b c Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ^ Polyanskiy, Mikhail. "Refractive index of LiIO3 (Lithium iodate) - Herbst-o". http://www.refractiveindex.info. Retrieved 2014-08-08.
{{cite web}}: External link in|website= - ^ a b Teyssier, Jeremie; Dantec, Ronan Le; Galez, Christine; Mugnier, Yannick; Bouillot, Jacques; Plenet, Jean-Claude (2003-11-20). "LiIO3 nanocrystals in SiO2 xerogels, a new material for non-linear optics". Proceeding SPIE. 5222 (26). doi:10.1117/12.507309.
- ^ a b c d Zachariasen, W.H.; Olof, F.A. BartaLars (1931-06-15). "Crystal Structure of Lithium Iodate". Physical Review Letters. 37: 1626. doi:10.1103/PhysRev.37.1626.
- ^ a b c Sigma-Aldrich Co., Lithium iodate. Retrieved on 2014-08-08.
- ^ "SDS of Lithium iodate anhydrous" (PDF). https://www.pfaltzandbauer.com. Connecticut, USA: Pfaltz & Bauer, Inc. Retrieved 2014-08-08.
{{cite web}}: External link in|website= - ^ Risk, W. P.; Gosnell, T. R.; Nurmikko, A. V. (9 January 2003). Compact Blue-Green Lasers. Cambridge University Press. p. 123. ISBN 978-0-521-52103-1. Retrieved 13 December 2012.
- ^ Nikogosyan, David N. (4 January 2005). Nonlinear Optical Crystals: A Complete Survey. Springer. p. 371. ISBN 978-0-387-22022-2. Retrieved 13 December 2012.

