Adenosine monophosphate: Difference between revisions
→Role in AMP-activated kinase regulation: Adding sources and fixed up grammar |
→cAMP: Added reference |
||
| Line 56: | Line 56: | ||
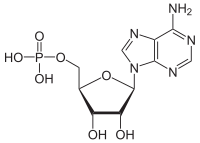
'''Adenosine monophosphate''' ('''AMP'''), also known as '''5'-adenylic acid''', is a [[nucleotide]]. AMP consists of a [[phosphate]] group, the sugar [[ribose]], and the nucleobase [[adenine]]; it is an [[ester]] of [[phosphoric acid]] and the nucleoside [[adenosine]]. As a [[substituent]] it takes the form of the prefix '''adenylyl-'''. |
'''Adenosine monophosphate''' ('''AMP'''), also known as '''5'-adenylic acid''', is a [[nucleotide]]. AMP consists of a [[phosphate]] group, the sugar [[ribose]], and the nucleobase [[adenine]]; it is an [[ester]] of [[phosphoric acid]] and the nucleoside [[adenosine]]. As a [[substituent]] it takes the form of the prefix '''adenylyl-'''. |
||
AMP plays an important role in many cellular metabolic processes, being interconverted to [[Adenosine diphosphate|ADP]] and/or [[Adenosine triphosphate|ATP]]. AMP is |
AMP plays an important role in many cellular metabolic processes, being interconverted to [[Adenosine diphosphate|ADP]] and/or [[Adenosine triphosphate|ATP]]. AMP is also a component in the synthesis of [[RNA]].<ref>{{Cite journal|last=Jauker|first=Mario|last2=Griesser|first2=Helmut|last3=Richert|first3=Clemens|date=2015-11-23|title=Spontaneous Formation of RNA Strands, Peptidyl RNA, and Cofactors|url=https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4678511/|journal=Angewandte Chemie (International Ed. in English)|volume=54|issue=48|pages=14564–14569|doi=10.1002/anie.201506593|issn=1433-7851|pmc=PMC4678511|pmid=26435376}}</ref> |
||
==Production and degradation== |
==Production and degradation== |
||
| Line 82: | Line 82: | ||
In a catabolic pathway, adenosine monophosphate can be converted to [[uric acid]], which is excreted from the body in mammals.<ref>{{Cite journal|date=2016-06-15|title=Regulation of uric acid metabolism and excretion|url=https://www.sciencedirect.com/science/article/pii/S0167527315303429|journal=International Journal of Cardiology|language=en|volume=213|pages=8–14|doi=10.1016/j.ijcard.2015.08.109|issn=0167-5273}}</ref> |
In a catabolic pathway, adenosine monophosphate can be converted to [[uric acid]], which is excreted from the body in mammals.<ref>{{Cite journal|date=2016-06-15|title=Regulation of uric acid metabolism and excretion|url=https://www.sciencedirect.com/science/article/pii/S0167527315303429|journal=International Journal of Cardiology|language=en|volume=213|pages=8–14|doi=10.1016/j.ijcard.2015.08.109|issn=0167-5273}}</ref> |
||
== Physiological Role == |
== Physiological Role in Regulation == |
||
=== Regulation === |
|||
=== AMP-activated kinase regulation === |
=== AMP-activated kinase regulation === |
||
| Line 94: | Line 92: | ||
==cAMP== |
==cAMP== |
||
AMP can also exist as a cyclic structure known as [[cyclic adenosine monophosphate|cyclic AMP]] (or cAMP). Within certain cells the enzyme [[adenylate cyclase]] makes cAMP from ATP, and typically this reaction is regulated by hormones such as [[adrenaline]] or [[glucagon]]. cAMP plays an important role in intracellular signaling. |
AMP can also exist as a cyclic structure known as [[cyclic adenosine monophosphate|cyclic AMP]] (or cAMP). Within certain cells the enzyme [[adenylate cyclase]] makes cAMP from ATP, and typically this reaction is regulated by hormones such as [[adrenaline]] or [[glucagon]]. cAMP plays an important role in intracellular signaling.<ref>{{Cite book|url=https://link.springer.com/chapter/10.1007/164_2015_32|title=Metabolic Control|last=Ravnskjaer|first=Kim|last2=Madiraju|first2=Anila|last3=Montminy|first3=Marc|date=2015|publisher=Springer, Cham|isbn=9783319298047|series=Handbook of Experimental Pharmacology|pages=29–49|language=en|doi=10.1007/164_2015_32}}</ref> |
||
==See also== |
==See also== |
||
Revision as of 02:46, 15 March 2018
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations. (February 2013) |
| |

| |
| Names | |
|---|---|
| IUPAC name
[(2R,3S,4R,5R)-5-(6-aminopurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate
| |
| Other names
Adenosine 5'-monophosphate, 5'-Adenylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.455 |
| KEGG | |
| MeSH | Adenosine+monophosphate |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H14N5O7P | |
| Molar mass | 347.22 g/mol |
| Appearance | white crystalline powder |
| Density | 2.32 g/mL |
| Melting point | 178 to 185 °C (352 to 365 °F; 451 to 458 K) |
| Boiling point | 798.5 °C (1,469.3 °F; 1,071.7 K) |
| Acidity (pKa) | 0.9[citation needed], 3.8, 6.1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Adenosine monophosphate (AMP), also known as 5'-adenylic acid, is a nucleotide. AMP consists of a phosphate group, the sugar ribose, and the nucleobase adenine; it is an ester of phosphoric acid and the nucleoside adenosine. As a substituent it takes the form of the prefix adenylyl-.
AMP plays an important role in many cellular metabolic processes, being interconverted to ADP and/or ATP. AMP is also a component in the synthesis of RNA.[1]
Production and degradation
AMP does not have the high energy phosphoanhydride bond associated with ADP and ATP. AMP can be produced from ADP:
- 2 ADP → ATP + AMP
Or AMP may be produced by the hydrolysis of one high energy phosphate bond of ADP:
- ADP + H2O → AMP + Pi
AMP can also be formed by hydrolysis of ATP into AMP and pyrophosphate:
- ATP + H2O → AMP + PPi
When RNA is broken down by living systems, nucleoside monophosphates, including adenosine monophosphate, are formed.
AMP can be regenerated to ATP as follows:
- AMP + ATP → 2 ADP (adenylate kinase in the opposite direction)
- ADP + Pi → ATP (this step is most often performed in aerobes by the ATP synthase during oxidative phosphorylation)
AMP can be converted into IMP by the enzyme myoadenylate deaminase, freeing an ammonia group.
In a catabolic pathway, adenosine monophosphate can be converted to uric acid, which is excreted from the body in mammals.[2]
Physiological Role in Regulation
AMP-activated kinase regulation
The eukaryotic cell enzyme 5' adenosine monophosphate-activated protein kinase, or AMPK, utilizes AMP for homeostatic energy processes during times of high cellular energy expenditure, such as exercise.[3] Since ATP cleavage, and corresponding phosphorylation reactions, are utilized in various processes throughout the body as a source of energy, ATP production is necessary to further create energy for those mammalian cells. AMPK, as a cellular energy sensor, is activated by decreasing levels of ATP, which is naturally accompanied by increasing levels of ADP and AMP.[4]
Though phosphorylation appears to be the main activator for AMPK, some studies suggest that AMP is an allosteric regulator and direct agonist for AMPK[5]. Furthermore, other studies suggest that the high ratio of AMP:ATP levels in cells, rather than just AMP, activate AMPK.[6]
AMP binds to the γ-subunit of AMPK, leading to the activation of the kinase, and then eventually a cascade of other processes such as the activation of catabolic pathways and inhibition of anabolic pathways to regenerate ATP. Catabolic mechanisms, which generate ATP through the release of energy from breaking down molecules, are activated by the AMPK enzyme while anabolic mechanisms, which utilize energy from ATP to form products, are inhibited.[7] Though the γ-subunit can bind AMP/ADP/ATP, only the binding of AMP/ADP results in a conformational shift of the enzyme protein. This variance in AMP/ADP versus ATP binding leads to a shift in the dephosphorylation state for the enzyme.[8] The dephosphorylation of AMPK through various protein phosphatases completely inactivates catalytic function. AMP/ADP protects AMPK from being inactivated by binding to the γ-subunit and maintaining the dephosphorylation state.[9]
cAMP
AMP can also exist as a cyclic structure known as cyclic AMP (or cAMP). Within certain cells the enzyme adenylate cyclase makes cAMP from ATP, and typically this reaction is regulated by hormones such as adrenaline or glucagon. cAMP plays an important role in intracellular signaling.[10]
See also
References
- ^ Jauker, Mario; Griesser, Helmut; Richert, Clemens (2015-11-23). "Spontaneous Formation of RNA Strands, Peptidyl RNA, and Cofactors". Angewandte Chemie (International Ed. in English). 54 (48): 14564–14569. doi:10.1002/anie.201506593. ISSN 1433-7851. PMC 4678511. PMID 26435376.
{{cite journal}}: CS1 maint: PMC format (link) - ^ "Regulation of uric acid metabolism and excretion". International Journal of Cardiology. 213: 8–14. 2016-06-15. doi:10.1016/j.ijcard.2015.08.109. ISSN 0167-5273.
- ^ Richter, Erik A.; Ruderman, Neil B. (2009-03-01). "AMPK and the biochemistry of exercise: Implications for human health and disease". The Biochemical journal. 418 (2): 261–275. doi:10.1042/BJ20082055. ISSN 0264-6021. PMC 2779044. PMID 19196246.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Carling, David; Mayer, Faith V; Sanders, Matthew J; Gamblin, Steven J (August 2011). "AMP-activated protein kinase: nature's energy sensor". Nature Chemical Biology. 7 (8): 512–518. doi:10.1038/nchembio.610. ISSN 1552-4469.
- ^ "The AMP-activated protein kinase (AMPK) and cancer: Many faces of a metabolic regulator". Cancer Letters. 356 (2): 165–170. 2015-01-28. doi:10.1016/j.canlet.2014.01.018. ISSN 0304-3835.
- ^ Hardie, D. Grahame (2011-09-15). "AMP-activated protein kinase—an energy sensor that regulates all aspects of cell function". Genes & Development. 25 (18): 1895–1908. doi:10.1101/gad.17420111. ISSN 0890-9369. PMID 21937710.
- ^ Hardie, D. Grahame (February 2011). "Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism". Proceedings of the Nutrition Society. 70 (1): 92–99. doi:10.1017/S0029665110003915. ISSN 1475-2719.
- ^ Krishan, Sukriti; Richardson, Des R.; Sahni, Sumit (2015-03-01). "Adenosine Monophosphate–Activated Kinase and Its Key Role in Catabolism: Structure, Regulation, Biological Activity, and Pharmacological Activation". Molecular Pharmacology. 87 (3): 363–377. doi:10.1124/mol.114.095810. ISSN 0026-895X. PMID 25422142.
- ^ Xiao, Bing; Sanders, Matthew J.; Underwood, Elizabeth; Heath, Richard; Mayer, Faith V.; Carmena, David; Jing, Chun; Walker, Philip A.; Eccleston, John F. (2011-04-14). "Structure of mammalian AMPK and its regulation by ADP". Nature. 472 (7342): 230–233. doi:10.1038/nature09932. ISSN 1476-4687. PMC 3078618. PMID 21399626.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Ravnskjaer, Kim; Madiraju, Anila; Montminy, Marc (2015). Metabolic Control. Handbook of Experimental Pharmacology. Springer, Cham. pp. 29–49. doi:10.1007/164_2015_32. ISBN 9783319298047.
External links
- GMD MS Spectrum
- Ming D, Ninomiya Y, Margolskee RF (1999). "Blocking taste receptor activation of gustducin inhibits gustatory responses to bitter compounds". Proc. Natl. Acad. Sci. USA. 96 (17): 9903–9908. doi:10.1073/pnas.96.17.9903. PMC 22308. PMID 10449792.
