Vanadium
 | |||||||||||||||||||||||||||||||
| Vanadium | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /vəˈneɪdiəm/ | ||||||||||||||||||||||||||||||
| Appearance | blue-silver-grey metal | ||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(V) | |||||||||||||||||||||||||||||||
| Vanadium in the periodic table | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
| Atomic number (Z) | 23 | ||||||||||||||||||||||||||||||
| Group | group 5 | ||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d3 4s2 | ||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 11, 2 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||
| Melting point | 2183 K (1910 °C, 3470 °F) | ||||||||||||||||||||||||||||||
| Boiling point | 3680 K (3407 °C, 6165 °F) | ||||||||||||||||||||||||||||||
| Density (at 20° C) | 6.099 g/cm3 [3] | ||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 5.5 g/cm3 | ||||||||||||||||||||||||||||||
| Heat of fusion | 21.5 kJ/mol | ||||||||||||||||||||||||||||||
| Heat of vaporization | 444 kJ/mol | ||||||||||||||||||||||||||||||
| Molar heat capacity | 24.89 J/(mol·K) | ||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||
| Oxidation states | −3, −1, 0, +1, +2, +3, +4, +5 (an amphoteric oxide) | ||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.63 | ||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||
| Atomic radius | empirical: 134 pm | ||||||||||||||||||||||||||||||
| Covalent radius | 153±8 pm | ||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) (cI2) | ||||||||||||||||||||||||||||||
| Lattice constant | a = 302.72 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||
| Thermal expansion | 8.77×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||
| Thermal conductivity | 30.7 W/(m⋅K) | ||||||||||||||||||||||||||||||
| Electrical resistivity | 197 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +255.0×10−6 cm3/mol (298 K)[4] | ||||||||||||||||||||||||||||||
| Young's modulus | 128 GPa | ||||||||||||||||||||||||||||||
| Shear modulus | 47 GPa | ||||||||||||||||||||||||||||||
| Bulk modulus | 160 GPa | ||||||||||||||||||||||||||||||
| Speed of sound thin rod | 4560 m/s (at 20 °C) | ||||||||||||||||||||||||||||||
| Poisson ratio | 0.37 | ||||||||||||||||||||||||||||||
| Mohs hardness | 6.7 | ||||||||||||||||||||||||||||||
| Vickers hardness | 628–640 MPa | ||||||||||||||||||||||||||||||
| Brinell hardness | 600–742 MPa | ||||||||||||||||||||||||||||||
| CAS Number | 7440-62-2 | ||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||
| Discovery | Andrés Manuel del Río[5] (1801) | ||||||||||||||||||||||||||||||
| First isolation | Henry Enfield Roscoe (1867) | ||||||||||||||||||||||||||||||
| Named by | Nils Gabriel Sefström (1830) | ||||||||||||||||||||||||||||||
| Isotopes of vanadium | |||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||
Vanadium (/vəˈneɪdiəm/) is a chemical element that has the symbol V and atomic number 23. It is a soft, silvery grey, ductile transition metal. The formation of a oxide layer makes the metal stable against oxidation. By anlysing the mineral vanadinite, Andrés Manuel del Río discovered it in 1801 and named it erythronium, but withdrew his claim four years later when it was (incorrectly) suggested that the mineral was actually lead chromate. The element was rediscovered in 1831 by Nils Gabriel Sefström, who named the element vanadium after the goddess of beauty Vanadis. Both names were attributed to the fact that the redox chemistry of vanadium yields compounds in a wide range of colors.
It occurs naturally in about 65 different minerals and in fossil fuel deposits. It is produced in China and Russia from steel smelter slag while other countries use heavy oil flue dust or produce it as byproduct of uranium mining. It is mainly used to produce speciality steel alloys such as high speed tool steels. The compound vanadium pentoxide is used as catalyst for the production of sulfuric acid. Vanadium is found in many organisms, and is used by some life forms as active center of enzymes.
History
A new element was originally discovered by Andrés Manuel del Río, a Spanish-born Mexican mineralogist, in 1801. The element was extracted from a sample of Mexican "brown lead" ore (now named vanadinite). He found that its salts exhibit a wide variety of colors, so he first named the element panchromium (Greek: all colors), but later renamed it erythronium, since most of the salts turned red when heated. The French chemist Hippolyte Victor Collet-Descotils incorrectly declared that del Río's new element was only impure chromium. Del Río thought himself to be mistaken and accepted the statement of the French chemist that was also backed by del Río's friend Baron Alexander von Humboldt.[6]

In 1831, Nils Gabriel Sefström of Sweden rediscovered the element in a new oxide which he found while working with some iron ores and later that same year Friedrich Wöhler confirmed del Río's earlier work.[7] Sefström choose a name beginning with V, which had not yet been assigned to any element, and called it vanadium after Vanadis (another name for Freya, the Scandinavian goddess of fertility), because of many the beautifully colored chemical compounds it produces.[7] In 1831, the geologist George William Featherstonhaugh suggested that vanadium should be renamed "rionium" after del Río, but this suggestion was not followed.[8]
The development of routes to pure vanadium metal spanned many years. In 1831, Berzelius reported the production of the metal, but Henry Enfield Roscoe showed that Berzelius had in fact produced the nitride, VN. Roscoe produced the metal in 1867 by reduction of vanadium(III) chloride, VCl3, with hydrogen.[9] In 1927 the pure vanadium was produced by reducing vanadium pentoxide with calcium.[10] The first large scale industrial use was in the chassis of the Ford Model T, which was inspired from French race cars. Vanadium steel allowed for reduced weight while simultaneously increasing tensile strength.[11]
Characteristics
Physical

Vanadium is a soft, ductile, silver-grey metal. It has good resistance to corrosion both by alkalis and sulfuric and hydrochloric acids.[13] It is oxidized in air at about 933 K (660 °C). Vanadium has good structural strength. Common oxidation states of vanadium include +2, +3, +4 and +5. In a popular experiment, ammonium vanadate (NH4VO3) can be successively reduced with zinc metal to demonstrate the different colours of vanadium in these four oxidation states. Lower oxidation states occur in compounds such as V(CO)6 and [V(CO)6]- and substituted derivatives.[13]
Isotopes
Naturally occurring vanadium is composed of one stable isotope 51V and one radioactive isotope 50V with a half-life of 1.5×1017 years and natural abundance 0.25%. 51V has a nuclear spin of 7/2.[14]
24 artificial radioisotopes have been characterized (in the range of mass number between 40 and 65). The most stable of these isotopes are 49V with a half-life of 330 days and 48V with a half-life of 15.9735 days. All of the remaining radioactive isotopes have half-lives shorter than an hour, the majority of them below 10 seconds. 4 isotopes have metastable excited states.[14]
Chemistry

The chemistry of vanadium is noteworthy for the ready accessibility of four adjacent oxidation states. Conversions of these oxidation states is illustrated by the reduction of a strongly acidic solution of a vanadium(V) compound with zinc dust. The initial yellow color characteristic of the vanadate ion, VO43−, is replaced by the blue color of [VO(H2O)5]2+, followed by the green color of [V(H2O)6]3+ and then violet, due to [V(H2O)6]2+.[13]
The most commercially important compound is vanadium pentoxide, which is used as a catalyst for the production of sulfuric acid.[13] This compound oxidizes sulfur dioxide to the trioxide. In this redox reaction, sulfur is oxidized from +4 to +6, and vanadium is reduced from +5 to +3:
- V2O5 + 2 SO2 → V2O3 + 2 SO3
The catalyst us regenerated by oxidation with air.
- V2O3 + O2 → V2O5
Several halides are known for oxidation states 2, 3 and 4. VCl4 is the most important commercially. This liquid is mainly used as a catalyst for polymerization of dienes. Vanadium(II) compounds are reducing agents, and vanadium(V) derivatives are oxidizing agents. Vanadium(IV) compounds often exist as vanadyl derivatives which contain the VO2+ center.[13]
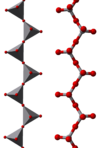
The oxyanion chemistry of vanadium(V) is complex. The vanadate ion, VO43−, is present in dilute solutions at high pH. On lowering the pH, HVO42- and H2VO4- are formed, analogous to HPO42- and H2PO4-. The acid dissociation constants for the vanadium and phosphorus series are remarkably similar. In more concentrated solutions many polyvanadates are formed. Chains, rings and clusters involving tetrahedral vanadium, analogous to the polyphosphates, are known. In addition, clusters such as the decavanadates V10O284− and HV10O283−, which predominate in the pH range 4-6, are formed in which the vanadium is octahedral.[13]
 |
 |
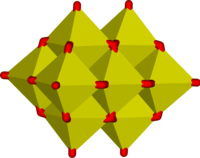 |
The correspondence between vanadate and phosphate chemistry can be attributed to the similarity in size and charge of phosphorus(V) and vanadium(V). Orthovanadate VO43− is used in protein crystallography to study the biochemistry of phosphate.
Coordination compounds
Three factors are rather unusual in the coordination chemistry of vanadium, due its position early on in the transition metal series. Firstly, metallic vanadium has the electronic configuration [Ar]4s23d3, so compounds of vanadium are relatively electron-poor. In consequence, most binary compounds are Lewis acids (electron pair acceptors). For example all the halides form octahedral adducts with the formula VXnL6-n (X = halide; L = other ligand). Secondly the vanadium ion is rather large and can achieve coordination numbers higher than 6, as in [V(CN)7]4−. Thirdly, the vanadyl ion, VO2+, features in many complexes of vanadium(IV). Vanadyl acetylacetonate is just one example. Interestingly, the vanadium is 5-coordinate, square pyramidal in this complex; a sixth ligand, such as pyridine, can be attached, but the equilibrium constant for that process is small. Many vanadyl complexes are 5-coordinate, but not all of them are square pyramidal. For example, VO(Cl2(NMe3)2 is trigonal bypyramidal.
Organometallic chemistry of vanadium is well developed, but organometallic compounds are of minor commercial significance. Vanadocene dichloride is a versatile starting reagent and even finds minor applications in organic chemistry.[15] Vanadium carbonyl, V(CO)6, is a rare example of an metal carbonyl that has an unpaired electron, but does not dimerize. Addition of an electron yields V(CO)6− which is isoelectronic with Cr(CO)6. Further reduction with sodium in liquid ammonia yields V(CO)53−, isoelectronic with Fe(CO)5.[16][17]
Occurrence

Metallic vanadium is not found in nature, but about 65 different minerals are known. Economically significant examples include patronite (VS4),[18] vanadinite (Pb5(VO4)3Cl), and carnotite (K2(UO2)2(VO4)2·3H2O). Much of the world's vanadium production is sourced from vanadium-bearing magnetite found in ultramafic gabbro bodies. Vanadium is mined mostly in South Africa, north west China and eastern Russia. In 2007 these three countries mined more than 95 % of the 58,600 tonnes of vanadium produced.[19]
Vanadium is also present in bauxite and in fossil fuel deposits such as crude oil, coal, oil shale and tar sands. In crude oil, concentrations up to 1200 ppm have been reported.[20] An estimated 110,000 tonnes per year enter the atmosphere by burning fussile fuel.[21] Vanadium has been detected spectroscopically in light from the Sun and some other stars.
Production
Industrially, most vanadium is used as ferrovanadium, an additive to improve steels. Ferrovanadium is produced directly by reducing a mixture of vanadium oxide, iron oxides and iron in an electric furnace. Vanadium-bearing magnetite iron ore is the main source for vanadium production.[22] The vanadium ends in pig iron produced from vanadium bearing magnetite. During steel production, oxygen is blown into the pig iron, oxidizing the carbon and most of the other impurities, forming slag. Depending on the used ore, the slag contains up to 25% of vanadium.[22]
Vanadium metal is obtained via a multistep process that begins with the roasting of crushed ore with NaCl or Na2CO3 at about 850 °C to give sodium metavanadate (NaVO3). An aqueous extract of this solid is acidified to give "red cake", a polyvanadate salt, which is reduced with calcium metal. As an alternative for small scale production, vanadium pentoxide is reduced with hydrogen or magnesium. Many other methods are also in use, in all of which vanadium is produced as a byproduct of other processes.[22]
Purification of vanadium is possible by the crystal bar process developed by Anton Eduard van Arkel and Jan Hendrik de Boer in 1925. It involves the formation of the metal iodide, in this example vanadium(III) iodide, and the subsequent decomposition to yield pure metal.
- 2 V + 3 I2 ⇌ 2 VI3
Applications
Alloys


Approximately 85% of vanadium produced is used as ferrovanadium or as a steel additive.[22] The considerable increase of strength in steel containing small amounts of vanadium was discovered in the beginning of 20th century,[23] and from that time vanadium steel was used for applications in axles, bicycle frames, crankshafts, gears, and other critical components. Vanadium forms stable nitrides and carbides, resulting in a significant increase in the strength of the steel. There are two groups of vanadium containing steel alloy groups. Vanadium high-carbon steel alloys containing 0.15 to 0.25 percent vanadium and high speed tool steels (HSS) with a vanadium content ranges from 1 % to 5 %. For the HSS after hardening a hardness above HRC60) can be achieved. HSS steel is used in surgical instruments and tools.[24]
Vanadium stabilizes the beta form of titanium and increases the strength and temperature stability. Mixed with aluminium in titanium alloys it is used in jet engines and high-speed airframes. One of the common alloys is Ti 6Al 4V a titanium alloy with 6% aluminium and 4% of vanadium.[25]
Other uses
- Vanadium foil is used in cladding titanium to steel.[26]
- Vanadium pentoxide, V2O5, is used as a catalyst in manufacturing sulfuric acid by the contact process[27] and as an oxidizer in maleic anhydride production.[28] It is also used in making ceramics.[29]
- Because of its moderate thermal neutron-capture cross-section and the short half-life of the isotopes produced by neutron capture, vanadium has been proposed as the material to be used for the inner structure of a fusion reactor.[30][31]
- Vanadium-gallium tape is used in superconducting magnets (17.5 teslas or 175,000 gauss). The structure of the superconducting A15 phase of V3Ga is similar to that of the more common Nb3Sn and Nb3Ti.[32] The first A15 phase superconductor was also a vanadium compound. V3Si, which was discovered in 1952.[33]
- Glass coated with vanadium dioxide VO2 can block infrared radiation (and not visible light) at a specific temperature.[34]
- vanadium redox rechargeable batteries employ vanadium redox couples in both half-cells, thereby eliminating the problem of cross contamination by diffusion of ions across the membrane.[35]
- Simulated alexandrite jewelry can be made by adding vanadium oxide to corundum.[36]
- Vanadate electrochemical conversion coatings are used for protecting steel against rust and corrosion.[37]
- Lithium vanadium oxide has been proposed for use as a high energy density anode for lithium ion batteries, at 745 Wh/l when paired with a lithium cobalt oxide cathode.[38]
- A small amount, 40 to 270 ppm, of vanadium in Wootz steel and Damascus steel, significantly improves the strength of the material.[39]
Biological role


Vanadium plays very limited role in biology. A vanadium-containing nitrogenase is used by some nitrogen-fixing micro-organisms. Vanadium is essential to ascidians or sea squirts in vanadium chromagen proteins. The concentration of vanadium in their blood is more than 100 times higher than the concentration of vanadium in the seawater around them. Rats and chickens are also known to require vanadium in very small amounts and deficiencies result in reduced growth and impaired reproduction.[40] Vanadium is a relatively controversial dietary supplement, primarily for increasing insulin sensitivity[41] and body-building. Whether it works for the latter purpose has not been proven, and there is some evidence that athletes who take it are merely experiencing a placebo effect.[42]
Ten percent of the blood cell pigment of the sea cucumber is vanadium. Just as the horseshoe crab has blue blood rather than red blood (colored by iron in hemoglobin) because of copper in the hemocyanin pigment, the blood of the sea cucumber is yellow because of the vanadium in the vanabin pigment.[43] Nonetheless, there is no evidence that vanabins carry oxygen, in contrast to hemoglobin and hemocyanin.
Vanadyl sulfate may improve glucose control in people with type 2 diabetes.[44][45][46][47][48]
Several species of macrofungi, namely Amanita muscaria and related species, accumulate vanadium (up to 500 mg/kg in dry weight). Vanadium is present in the coordination complex amavadine in fungal fruit-bodies. However, the biological importance of the accumulation process is unknown.[49][50]
Health and safety
All vanadium compounds should be considered to be toxic. Tetravalent VOSO4 has been reported to be over 5 times more toxic than trivalent V2O3.[51] The most dangerous compound is vanadium pentoxide. The Occupational Safety and Health Administration (OSHA) has set an exposure limit of 0.05 mg/m3 for vanadium pentoxide dust and 0.1 mg/m3 for vanadium pentoxide fumes in workplace air for an 8-hour workday, 40-hour work week. The National Institute for Occupational Safety and Health (NIOSH) has recommended that 35 mg/m3 of vanadium be considered immediately dangerous to life and health. This is the exposure level of a chemical that is likely to cause permanent health problems or death.
Vanadium compounds are poorly absorbed through the gastrointestinal system. Inhalation exposures to vanadium and vanadium compounds result primarily in adverse effects on the respiratory system.[52][53][54] Quantitative data are, however, insufficient to derive a subchronic or chronic inhalation reference dose. Other effects have been reported after oral or inhalation exposures on blood parameters,[55][56] on liver,[57] on neurological development in rats,[58] and other organs.
There is little evidence that vanadium or vanadium compounds are reproductive toxins or teratogens. Vanadium pentoxide was reported to be carcinogenic in male rats and male and female mice by inhalation in an NTP study,[59] although the interpretation of the results has recently been disputed.[60] Vanadium has not been classified as to carcinogenicity by the U.S. EPA.[61]
Metallic vanadium is potentially a fire hazard, particularly when in a finely-divided state.
Vanadium in literature
Vanadium naphthenate has a curious place in literature. It features in the short story, entitled “Vanadium” in Primo Levi’s book, The Periodic Table.[62] It was instrumental in bringing Levi into correspondence, after the war, with a German chemist he had encountered when working as a slave-labourer in the Buna-Monowitz factory at Auschwitz.
References
- ^ "Standard Atomic Weights: Vanadium". CIAAW. 1977.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ "Vanadium". Royal Society of Chemistry. Royal Society of Chemistry. Retrieved December 5, 2022.
- ^ Pedro Cintas (2004). "The Road to Chemical Names and Eponyms: Discovery, Priority, and Credit". Angewandte Chemie International Edition. 43 (44): 5888–5894. doi:10.1002/anie.200330074.
- ^ a b Sefström, N. G. (1831). "Ueber das Vanadin, ein neues Metall, gefunden im Stangeneisen von Eckersholm, einer Eisenhütte, die ihr Erz von Taberg in Småland bezieht". Annalen der Physik und Chemie. 97 (1): 43–49. doi:10.1002/andp.18310970103.
- ^ Featherstonhaugh, George William (1831). The Monthly American Journal of Geology and Natural Science: 69.
- ^ Henry E. Roscoe (1869 – 1870). "Researches on Vanadium.--Part II". Proceedings of the Royal Society of London. 18: 37–42. doi:10.1098/rspl.1869.0012.
{{cite journal}}: Check date values in:|year=(help) - ^ Marden, J. W. (1927). "Vanadium". Industrial and Engineering Chemistry. 19 (7): 786–788. doi:10.1021/ie50211a012.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Betz, Frederick (2003). Managing Technological Innovation: Competitive Advantage from Change. Wiley-IEEE. pp. 158–159. ISBN 0471225630.
- ^ Al-Kharafi, F. M. (1997). "Electrochemical behaviour of vanadium in aqueous solutions of different pH". Electrochimica Acta. 42 (4): 579–586. doi:10.1016/S0013-4686(96)00202-2.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f Holleman, Arnold F. (1985). "Vanadium". Lehrbuch der Anorganischen Chemie (in German) (91–100 ed.). Walter de Gruyter. pp. 1071–1075. ISBN 3110075113.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ a b Georges, Audi (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3–128. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ G. Wilkinson and J.G. Birmingham (1954). "Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta". Journal of the American Chemical Society. 76 (17): 4281–4284. doi:10.1021/ja01646a008.
- ^ Bellard, S. (1979). "Crystal and molecular structure of vanadium hexacarbonyl". Acta Crystallographica. B35: 271–274. doi:10.1107/S0567740879003332.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Elschenbroich, C. (1992). Organometallics : A Concise Introduction. Wiley-VCH. ISBN 3527281657.
{{cite book}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "mineralogical data about Patrónite". mindata.org. Retrieved 2009-01-19.
- ^ Magyar, Michael J. "Mineral Commodity Summaries 2008: Vanadium" (PDF). United States Geological Survey. Retrieved 2009-01-15.
- ^ Pearson, C. D. (1993). "Vanadium and nickel complexes in petroleum resid acid, base, and neutral fractions". Energy Fuels. 7 (3 pages = 338–346): 338. doi:10.1021/ef00039a001.
{{cite journal}}: Missing pipe in:|issue=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Anke, Manfred (2004). "Vanadium - An element both essential and toxic to plants, animals and humans?" (PDF). Anal. Real Acad. Nac. Farm. 70: 961–999.
- ^ a b c d Moskalyk, R. R. (2003). "Processing of vanadium: a review". Minerals Engineering. 16 (9, September 2003): 793–805. doi:10.1016/S0892-6875(03)00213-9.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Chandler, Harry (1998). Metallurgy for the Non-metallurgist. ASM International. pp. 6–7. ISBN 9780871706522.
{{cite book}}: Unknown parameter|yera=ignored (help) - ^ Davis, Joseph R. (1995). Tool Materials: Tool Materials. ASM International. ISBN 9780871705457.
- ^ Peters, Manfred (2002). "Metastabile β-Legierungen". Titan und Titanlegierungen. Wiley-VCH. pp. 23–24. ISBN 9783527305391.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lositskii, N. T. (1966). "Welding of chemical equipment made from two-layer sheet with titanium protective layer (review of foreign literature)". Chemical and Petroleum Engineering. 2 (12): 854–856. doi:10.1007/BF01146317.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Eriksen, K. M. (1995). "Deactivation and Compound Formation in Sulfuric-Acid Catalysts and Model Systems". Journal of Catalysis. 155 (1): 32–42. doi:10.1006/jcat.1995.1185.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Abon, Michel (1997). "Vanadium phosphorus oxides for n-butane oxidation to maleic anhydride". Applied Catalysis A: General. 157: 173–193. doi:10.1016/S0926-860X(97)00016-1.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|issues=ignored (help) - ^ Lide, David R. (2004). "vanadium". CRC Handbook of Chemistry and Physics. pp. 4–34. ISBN 9780849304859.
{{cite book}}: Text "CRC Press" ignored (help) - ^ Matsui, H. (1996). "Status of vanadium alloys for fusion reactors". Journal of Nuclear Materials. 233–237 (1): 92–99. doi:10.1016/S0022-3115(96)00331-5.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ "Vanadium Data Sheet" (PDF). Allegheny Technologies – Wah Chang. Retrieved 2009-01-16.
- ^ Markiewicz, W. (1977). "A 17.5 Tesla superconducting concentric Nb3Sn and V3Ga magnet system". IEEE Transactions on Magnetics. 13 (1): 35–37. doi:10.1109/TMAG.1977.1059431.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hardy, George F. (1953). "Superconducting Silicides and Germanides". Physical Reviews. 89: 884–884. doi:10.1103/PhysRev.89.884.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Manning, Troy D. (2002). "Intelligent window coatings: atmospheric pressure chemical vapour
deposition of vanadium oxides". Journal of Materials Chemistry. 12: 2936–2939. doi:10.1039/b205427m.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); line feed character in|title=at position 66 (help) - ^ Joerissen, Ludwig (2004). "Possible use of vanadium redox-flow batteries for energy storage in small grids and stand-alone photovoltaic systems". Journal of Power Sources. 127: 98–104. doi:10.1016/j.jpowsour.2003.09.066.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|issues=ignored (help) - ^ White, Willam B. (1962). "The Alexandrite Effect: And Optical Study" (PDF). American Mineralogist. 52: 867–871.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Guan, H. (2004). "Corrosion Protection of Aluminum Alloy 2024-T3 by Vanadate Conversion Coatings". Corrosion. 60 (3): 284–296.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kariatsumari, Koji (February 2008). "Li-Ion Rechargeable Batteries Made Safer". Nikkei Business Publications, Inc. Retrieved 10-12-2008.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Verhoeven, J. D. (1998). "The key role of impurities in ancient damascus steel blades". Journal of the Minerals, Metals and Materials Society. 50 (9): 58–64. doi:10.1007/s11837-998-0419-y.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Schwarz, Klaus (1971). "Growth Effects of Vanadium in the Rat". Science. 174 (4007): 426–428.
{{cite journal}}: Unknown parameter|coauthor=ignored (|author=suggested) (help) - ^ Yeh, Gloria Y. (2003). "Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes". Diabetes Care. 26: 1277–1294.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Talbott,, Shawn M. (2007). "Vanadium". The Health Professional's Guide to Dietary Supplements. Lippincott Williams & Wilkins. pp. 419–422. ISBN 9780781746724.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: extra punctuation (link) - ^ Natkin, Michael (2007). "Blood Color". Science Facts. Soak (Source Of All Knowledge). Retrieved 2007-11-16.
- ^ Halberstam, M; et al. (1996). "Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects". Diabetes. 45: 659–66. doi:10.2337/diabetes.45.5.659. PMID 8621019.
{{cite journal}}: Explicit use of et al. in:|first=(help) - ^ Boden, G; et al. (1996;). "Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin dependent diabetes mellitus". Metabolism. 45: 1130–5. doi:10.1016/S0026-0495(96)90013-X.
{{cite journal}}: Check date values in:|year=(help); Explicit use of et al. in:|first=(help)CS1 maint: extra punctuation (link) - ^ Goldfine, AB; et al. (2000). "Metabolic effects of vanadyl sulfate in humans with non-insulin-dependent diabetes mellitus: in vivo and in vitro studies". Metabolism. 49: 400–10. doi:10.1016/S0026-0495(00)90418-9.
{{cite journal}}: Explicit use of et al. in:|first=(help) - ^ Badmaev, V; et al. (1999). "Vanadium: a review of its potential role in the fight against diabetes". Altern Complement Med. 5: 273–291. doi:10.1089/acm.1999.5.273.
{{cite journal}}: Explicit use of et al. in:|first=(help) - ^ Goldwaser, I; et al. (1999). "L-Glutamic Acid gamma -Monohydroxamate. A Potentiator of Vanadium-Evoked Glucose Metabolism in vitro and in vivo". J Biol Chem. 274: 26617–26624. doi:10.1074/jbc.274.37.26617. PMID 10473627.
{{cite journal}}: Explicit use of et al. in:|first=(help)CS1 maint: unflagged free DOI (link) - ^ Falandysz, J. (2007). "Selected elements in fly agaric Amanita muscaria". Journal of Environmental Science and Health, Part A. 42 (11): 1615–1623. doi:10.1080/10934520701517853.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Berry, Robert E. (1999). "Die Struktur von Amavadin". Angewandte Chemie. 111 (6): 871–873. doi:10.1002/(SICI)1521-3757(19990315)111:6<871::AID-ANGE871>3.0.CO;2-#.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Roschin, A. V. (1967). "Toxicology of vanadium compounds used in modern industry". Gig Sanit. (Water Res.). 32: 26–32.
{{cite journal}}: Unknown parameter|pimid=ignored (help) - ^ Sax, N. I. (1984). Dangerous Properties of Industrial Materials, 6th ed. Van Nostrand Reinhold Company. pp. 2717–2720.
- ^ N. B. Ress*, B. J. Chou, R. A. Renne, J. A. Dill, R. A. Miller, J. H. Roycroft, J. R. Hailey, J. K. Haseman and J. R. Bucher (2003). "Carcinogenicity of Inhaled Vanadium Pentoxide in F344/N Rats and B6C3F1 Mice". Toxicological Sciences. 74: 2876–296. PMID 12773761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jörg M. Wörle-Knirsch, Katrin Kern, Carsten Schleh, Christel Adelhelm, Claus Feldmann, and Harald F. Krug (2007). "Nanoparticulate Vanadium Oxide Potentiated Vanadium Toxicity in Human Lung Cells". Environ. Sci. Technol. 41: 331–336. doi:10.1021/es061140x.
{{cite journal}}: Unknown parameter|issues=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ścibior, A. (2006). "Selected haematological and biochemical parameters of blood in rats after subchronic administration of vanadium and/or magnesium in drinking water". Archives of Environmental Contamination and Toxicology. 51 (2): 287–295. doi:10.1007/s00244-005-0126-4.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Gonzalez-Villalva, A. (2006). "Thrombocytosis induced in mice after subacute and subchronic V2O5 inhalation". Toxicology and Industrial Health. 22 (3): 113–116. doi:10.1191/0748233706th250oa. PMID 16716040.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Kazuo Kobayashia, Seiichiro Himeno, Masahiko Satoh, Junji Kuroda, Nobuo Shibata, Yoshiyuki Seko and Tatsuya Hasegawa (2006,). "Pentavalent vanadium induces hepatic metallothionein through interleukin-6-dependent and -independent mechanisms". Toxicology. 228: 162–170. doi:10.1016/j.tox.2006.08.022.
{{cite journal}}: Check date values in:|year=(help); Unknown parameter|issues=ignored (help)CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Soazo, Marina (2007). "Vanadium exposure through lactation produces behavioral alterations and CNS myelin deficit in neonatal rats". Neurotoxicology and Teratology. 29 (4): 503–510. doi:10.1016/j.ntt.2007.03.001.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ress, N. B. (2003). "Carcinogenicity of inhaled vanadium pentoxide in F344/N rats and B6C3F1 mice". Toxicological Sciences. 74 (2): 287–296. doi:10.1093/toxsci/kfg136. PMID 12773761.
{{cite journal}}: Cite has empty unknown parameter:|month=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Duffus, J. H. (2007). "Carcinogenicity classification of vanadium pentoxide and inorganic vanadium compounds, the NTP study of carcinogenicity of inhaled vanadium pentoxide, and vanadium chemistry". Regulatory Toxicology and Pharmacology. 47 (1): 110–114. doi:10.1016/j.yrtph.2006.08.006.
{{cite journal}}: Cite has empty unknown parameters:|month=and|coauthors=(help) - ^ Opreskos, Dennis M. (1991). "Toxicity Summary for Vanadium". Oak Ridge National Laboratory. Retrieved 2008-11-08.
- ^ Levi, P. (2000). The Periodic table. Rosenthal, R. (translator). Penguin Classics. ISBN 0141185147.
External links
- The periodic table of videos videos of the chemistry of the elements
- Los Alamos National Laboratory – Vanadium
- WebElements.com – Vanadium
- ATSDR – ToxFAQs: Vanadium


