Nuclear fuel cycle
The nuclear fuel cycle, also called nuclear fuel chain, is the progression of nuclear fuel through a series of differing stages. It consists of steps in the front end, which are the preparation of the fuel, steps in the service period in which the fuel is used during reactor operation, and steps in the back end, which are necessary to safely manage, contain, and either reprocess or dispose of spent nuclear fuel. If spent fuel is not reprocessed, the fuel cycle is referred to as an open fuel cycle (or a once-through fuel cycle); if the spent fuel is reprocessed, it is referred to as a closed fuel cycle.
Fuel cycles
Once-through nuclear fuel cycle

Not a cycle per se, fuel is used once and then sent to storage without further processing save additional packaging to provide for better isolation from the biosphere. This method is favored by six countries: the United States, Canada, Sweden, Finland, Spain and South Africa.[1] Some countries, notably Sweden and Canada, have designed repositories to permit future recovery of the material should the need arise, while others plan for permanent sequestration in a geological repository like the Yucca Mountain nuclear waste repository in the United States.
Plutonium cycle
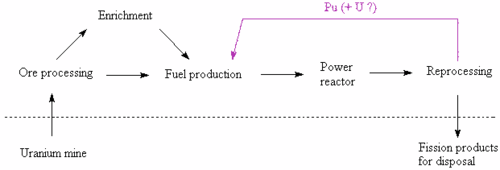
Several countries are using the reprocessing services offered by BNFL and COGEMA. Here, the fission products, minor actinides, activation products, and reprocessed uranium are separated from the reactor-grade plutonium, which can then be fabricated into MOX fuel. Because the proportion of the non-fissile even-mass isotopes of plutonium rises with each pass through the cycle, there are currently no plans to reuse plutonium from used MOX fuel for a third pass in a thermal reactor. However, if fast reactors become available, they may be able to burn these, or almost any other actinide isotopes.
Minor actinides recycling
It has been proposed that in addition to the use of plutonium, the minor actinides could be used in a critical power reactor. Tests are already being conducted in which americium is being used as a fuel.[2]
A number of reactor designs, like the Integral Fast Reactor, have been designed for this rather different fuel cycle. In principle, it should be possible to derive energy from the fission of any actinide nucleus. With a careful reactor design, all the actinides in the fuel can be consumed, leaving only lighter elements with short half-lives. Whereas this has been done in prototype plants, no such reactor has ever been operated on a large scale, and the first plants with full actinide recovery are expected to be ready for commercial deployment in 2015 at the earliest.
However, such schemes would most likely require advanced remote reprocessing methods due to the neutron emitting compounds formed. For instance if curium is irradiated with neutrons it will form the very heavy actinides californium and fermium which undergo spontaneous fission. As a result, the neutron emission from a used fuel element which had included curium will be much higher, potentially posing a risk to workers at the back end of the cycle unless all reprocessing is done remotely. This could be seen as a disadvantage, but on the other hand it also makes the nuclear material difficult to steal or divert, making it more resistant to nuclear proliferation
It so happens that the neutron cross-section of many actinides decreases with increasing neutron energy, but the ratio of fission to simple activation (neutron capture) changes in favour of fission as the neutron energy increases. Thus with a sufficiently high neutron energy, it should be possible to destroy even curium without the generation of the transcurium metals. This could be very desirable as it would make it significantly easier to reprocess and handle the actinide fuel.
One promising alternative from this perspective is an accelerator driven subcritical reactor. Here a beam of either protons (United States and European designs)[3][4][5] or electrons (Japanese design)[6] is directed into a target. In the case of protons, very fast neutrons will spall off the target, while in the case of the electrons, very high energy photons will be generated. These high-energy neutrons and photons will then be able to cause the fission of the heavy actinides.
Such reactors compare very well to other neutron sources in terms of neutron energy:
- Thermal 0 to 100 eV
- Epithermal 100 eV to 100 KeV
- Fast (from nuclear fission) 100 KeV to 3 MeV
- DD fusion 2.5 MeV
- DT fusion 14 MeV
- Accelerator driven core 200 MeV (lead driven by 1.6 GeV protons)
- Muon-catalyzed fusion 7 GeV.
As an alternative, the curium-244, with a half life of 18 years, could be left to decay into plutonium-240 before being used in fuel in a fast reactor.

Fuel or targets for this actinide transmutation
To date the nature of the fuel (targets) for actinide transformation has not been chosen.
If actinides are transmuted in a Subcritical reactor it is likely that the fuel will have to be able to tolerate more thermal cycles than conventional fuel. An accelerator driven sub critical reactor is unlikely to be able to maintain a constant operation period for equally long times as a critical reactor, and each time the accelerator stops then the fuel will cool down.
On the other hand, if actinides are destroyed using a fast reactor, such as an Integral Fast Reactor, then the fuel will most likely not be exposed to many more thermal cycles than in a normal power station.
Depending on the matrix the process can generate more transuranics from the matrix. This could either be viewed as good (generate more fuel) or can be viewed as bad (generation of more radiotoxic transuranic elements). A series of different matrices exist which can control this production of heavy actinides.
Fissile nuclei, like Uranium-235, Plutonium-239 and Uranium-233 respond well to delayed neutrons and are thus important to keep a critical reactor stable, and this limits the amount of minor actinides that can be destroyed in a critical reactor. As a consequence it is important that the chosen matrix allows the reactor to keep the ratio of fissile to non-fissile nuclei high, as this enables it to destroy the long lived actinides safely. In contrast, the power output of a sub-critical reactor is limited by the intensity of the driving particle accelerator, and thus it need not contain any uranium or plutonium at all. In such a system it may be preferable to have an inert matrix that doesn't produce additional long-lived isotopes.
Actinides in an inert matrix
The actinides will be mixed with a metal which will not form more actinides, for instance an alloy of actinides in a solid such as zirconia could be used.
Actinides in a thorium matrix
Thorium will on neutron bombardment form uranium-233. U-233 is fissile, and has a larger fission cross section than both U-235 and U-238, and thus it is likely to produce very little additional actinides through neutron capture.
Actinides in a uranium matrix
If the actinides are incorporated into a uranium-metal or uranium-oxide matrix, then the neutron capture of U-238 is likely to generate new plutonium-239. An advantage of mixing the actinides with uranium and plutonium is that the large fission cross sections of U-235 and Pu-239 for the less energetic delayed-neutrons could make the reaction stable enough to be carried out in a critical fast reactor, which is likely to be both cheaper and simpler than an accelerator driven system.
Mixed matrix
It is also possible to create a matrix made from a mix of the above mentioned materials. This is most commonly done in fast reactors where one may wish to keep the breeding ratio of new fuel high enough to keep powering the reactor, but still low enough that the generated actinides can be safely destroyed without transporting them to another site. One way to do this is to use fuel where actinides and uranium is mixed with inert zirconium, producing fuel elements with the desired properties.
Thorium cycle
In the thorium fuel cycle thorium-232 absorbs a neutron in either a fast or thermal reactor. The thorium-233 beta decays to protactinium-233 and then to uranium-233, which in turn is used as fuel. Hence, like uranium-238, thorium-232 is a fertile material.
After starting the reactor with existing U-233 or some other fissile material such as U-235 or Pu-239, a breeding cycle similar to but more efficient[7] than that with U-238 and plutonium can be created. The Th-232 absorbs a neutron to become Th-233 which quickly decays to protactinium-233. Protactinium-233 in turn decays with a half-life of 27 days to U-233. In some molten salt reactor designs, the Pa-233 is extracted and protected from neutrons (which could transform it to Pa-234 and then to U-234), until it has decayed to U-233. This is done in order to improve the breeding ratio which is low compared to fast reactors.
Thorium is at least 4-5 times more abundant in nature than all of uranium isotopes combined; thorium is fairly evenly spread around Earth with a lot of countries[8] having huge supplies of it; preparation of thorium fuel does not require difficult [7] and expensive enrichment processes; the thorium fuel cycle creates mainly Uranium-233 contaminated with Uranium-232 which makes it harder to use in a nuclear weapon; elimination of at least the transuranic portion of the nuclear waste problem is possible in MSR and other breeder reactor designs.
One of the earliest efforts to use a thorium fuel cycle took place at Oak Ridge National Laboratory in the 1960s. An experimental reactor was built based on molten salt reactor technology to study the feasibility of such an approach, using thorium fluoride salt kept hot enough to be liquid, thus eliminating the need for fabricating fuel elements. This effort culminated in the Molten-Salt Reactor Experiment that used 232Th as the fertile material and 233U as the fissile fuel. Due to a lack of funding, the MSR program was discontinued in 1976.
Current industrial activity
Currently the only isotopes used as nuclear fuel are uranium-235 (U-235), uranium-238 (U-238) and plutonium-239, although the proposed thorium fuel cycle has advantages. Some modern reactors, with minor modifications, can use thorium. Thorium is approximately three times more abundant in the Earth's crust than uranium (and 550 times more abundant than uranium-235). However, there has been little exploration for thorium resources, and thus the proved resource is small. Thorium is more plentiful than uranium in some countries, notably India.[9]
Heavy water reactors and graphite-moderated reactors can use natural uranium, but the vast majority of the world's reactors require enriched uranium, in which the ratio of U-235 to U-238 is increased. In civilian reactors the enrichment is increased to as much as 5% U-235 and 95% U-238, but in naval reactors there is as much as 93% U-235.
The term nuclear fuel is not normally used in respect to fusion power, which fuses isotopes of hydrogen into helium to release energy.
Front end
-
1 Uranium ore - the principal raw material of nuclear fuel
-
2 Yellowcake - the form in which uranium is transported to a conversion plant
-
3 UF6 - used in enrichment
-
4 Nuclear fuel - a compact, inert, insoluble solid
Exploration
A deposit of uranium, such as uraninite, discovered by geophysical techniques, is evaluated and sampled to determine the amounts of uranium materials that are extractable at specified costs from the deposit. Uranium reserves are the amounts of ore that are estimated to be recoverable at stated costs. Uranium in nature consists primarily of two isotopes, U-238 and U-235. The numbers refer to the atomic mass number for each isotope, or the number of protons and neutrons in the atomic nucleus. Naturally occurring uranium consists of approximately 99.28% U-238 and 0.71% U-235. The atomic nucleus of U-235 will nearly always fission when struck by a free neutron, and the isotope is therefore said to be a "fissile" isotope. The nucleus of a U-238 atom on the other hand, rather than undergoing fission when struck by a free neutron, will nearly always absorb the neutron and yield an atom of the isotope U-239. This isotope then undergoes natural radioactive decay to yield Pu-239, which, like U-235, is a fissile isotope. The atoms of U-238 are said to be fertile, because, through neutron irradiation in the core, some eventually yield atoms of fissile Pu-239.
Mining
Uranium ore can be extracted through conventional mining in open pit and underground methods similar to those used for mining other metals. In-situ leach mining methods also are used to mine uranium in the United States. In this technology, uranium is leached from the in-place ore through an array of regularly spaced wells and is then recovered from the leach solution at a surface plant. Uranium ores in the United States typically range from about 0.05 to 0.3% uranium oxide (U3O8). Some uranium deposits developed in other countries are of higher grade and are also larger than deposits mined in the United States. Uranium is also present in very low-grade amounts (50 to 200 parts per million) in some domestic phosphate-bearing deposits of marine origin. Because very large quantities of phosphate-bearing rock are mined for the production of wet-process phosphoric acid used in high analysis fertilizers and other phosphate chemicals, at some phosphate processing plants the uranium, although present in very low concentrations, can be economically recovered from the process stream.
Milling
Mined uranium ores normally are processed by grinding the ore materials to a uniform particle size and then treating the ore to extract the uranium by chemical leaching. The milling process commonly yields dry powder-form material consisting of natural uranium, "yellowcake", which is sold on the uranium market as U3O8.
Uranium conversion
Milled uranium oxide, U3O8, must be converted to uranium hexafluoride, UF6, which is the form required by most commercial uranium enrichment facilities currently in use. A solid at room temperature, uranium hexafluoride can be changed to a gaseous form at moderately higher temperature of 57 °C (134 °F). The uranium hexafluoride conversion product contains only natural, not enriched, uranium.
Triuranium octaoxide (U3O8) is also converted directly to ceramic grade uranium dioxide (UO2) for use in reactors not requiring enriched fuel, such as CANDU. The volumes of material converted directly to UO2 are typically quite small compared to the amounts converted to UF6.
Enrichment

The concentration of the fissionable isotope, U-235 (0.71% in natural uranium) is less than that required to sustain a nuclear chain reaction in light water reactor cores. Natural UF6 thus must be enriched in the fissionable isotope for it to be used as nuclear fuel. The different levels of enrichment required for a particular nuclear fuel application are specified by the customer: light-water reactor fuel normally is enriched to 3.5% U-235, but uranium enriched to lower concentrations is also required. Enrichment is accomplished using one or more methods of isotope separation. Gaseous diffusion and gas centrifuge are the commonly used uranium enrichment technologies, but new enrichment technologies are currently being developed.
The bulk (96%) of the byproduct from enrichment is depleted uranium (DU), which can be used for armor, kinetic energy penetrators, radiation shielding and ballast. Still, there are vast quantities of depleted uranium in storage. The United States Department of Energy alone has 470,000 tonnes.[10] About 95% of depleted uranium is stored as uranium hexafluoride (UF6).
Fabrication
For use as nuclear fuel, enriched uranium hexafluoride is converted into uranium dioxide (UO2) powder that is then processed into pellet form. The pellets are then fired in a high temperature sintering furnace to create hard, ceramic pellets of enriched uranium. The cylindrical pellets then undergo a grinding process to achieve a uniform pellet size. The pellets are stacked, according to each nuclear reactor core's design specifications, into tubes of corrosion-resistant metal alloy. The tubes are sealed to contain the fuel pellets: these tubes are called fuel rods. The finished fuel rods are grouped in special fuel assemblies that are then used to build up the nuclear fuel core of a power reactor.
The metal used for the tubes depends on the design of the reactor. Stainless steel was used in the past, but most reactors now use zirconium. For the most common types of reactors, boiling water reactors (BWR) and pressurized water reactors (PWR), the tubes are assembled into bundles[11] with the tubes spaced precise distances apart. These bundles are then given a unique identification number, which enables them to be tracked from manufacture through use and into disposal.
Service period
Transport of radioactive materials
Transport is an integral part of the nuclear fuel cycle. There are nuclear power reactors in operation in several countries but uranium mining is viable in only a few areas. Also, in the course of over forty years of operation by the nuclear industry, a number of specialized facilities have been developed in various locations around the world to provide fuel cycle services and there is a need to transport nuclear materials to and from these facilities. Most transports of nuclear fuel material occur between different stages of the cycle, but occasionally a material may be transported between similar facilities. With some exceptions, nuclear fuel cycle materials are transported in solid form, the exception being uranium hexafluoride (UF6) which is considered a gas. Most of the material used in nuclear fuel is transported several times during the cycle. Transports are frequently international, and are often over large distances. Nuclear materials are generally transported by specialized transport companies.
Since nuclear materials are radioactive, it is important to ensure that radiation exposure of both those involved in the transport of such materials and the general public along transport routes is limited. Packaging for nuclear materials includes, where appropriate, shielding to reduce potential radiation exposures. In the case of some materials, such as fresh uranium fuel assemblies, the radiation levels are negligible and no shielding is required. Other materials, such as spent fuel and high-level waste, are highly radioactive and require special handling. To limit the risk in transporting highly radioactive materials, containers known as spent nuclear fuel shipping casks are used which are designed to maintain integrity under normal transportation conditions and during hypothetical accident conditions.
In-core fuel management
A nuclear reactor core is composed of a few hundred "assemblies", arranged in a regular array of cells, each cell being formed by a fuel or control rod surrounded, in most designs, by a moderator and coolant, which is water in most reactors.
Because of the fission process that consumes the fuels, the old fuel rods must be changed periodically to fresh ones (this period is called a cycle). However, only a part of the assemblies (typically one-third) are removed since the fuel depletion is not spatially uniform. Furthermore, it is not a good policy, for efficiency reasons, to put the new assemblies exactly at the location of the removed ones. Even bundles of the same age may have different burn-up levels, which depends on their previous positions in the core. Thus the available bundles must be arranged in such a way that the yield is maximized, while safety limitations and operational constraints are satisfied. Consequently reactor operators are faced with the so-called optimal fuel reloading problem, which consists in optimizing the rearrangement of all the assemblies, the old and fresh ones, while still maximizing the reactivity of the reactor core so as to maximise fuel burn-up and minimise fuel-cycle costs.
This is a discrete optimization problem, and computationally infeasible by current combinatorial methods, due to the huge number of permutations and the complexity of each computation. Many numerical methods have been proposed for solving it and many commercial software packages have been written to support fuel management. This is an on-going issue in reactor operations as no definitive solution to this problem has been found and operators use a combination of computational and empirical techniques to manage this problem.
The study of used fuel
Used nuclear fuel is studied in Post irradiation examination, where used fuel is examined to know more about the processes that occur in fuel during use, and how these might alter the outcome of an accident. For example, during normal use, the fuel expands due to thermal expansion, which can cause cracking. Most nuclear fuel is uranium dioxide, which is a cubic solid with a structure similar to that of calcium fluoride. In used fuel the solid state structure of most of the solid remains the same as that of pure cubic uranium dioxide. SIMFUEL is the name given to the simulated spent fuel which is made by mixing finely ground metal oxides, grinding as a slurry, spray drying it before heating in hydrogen/argon to 1700 oC.[12] In SIMFUEL, 4.1% of the volume of the solid was in the form of metal nanoparticles which are made of molybdenum, ruthenium, rhodium and palladium. Most of these metal particles are of the ε phase (hexagonal) of Mo-Ru-Rh-Pd alloy, while smaller amounts of the α (cubic) and σ (tetragonal) phases of these metals were found in the SIMFUEL. Also present within the SIMFUEL was a cubic perovskite phase which is a barium strontium zirconate (BaxSr1-xZrO3).

Uranium dioxide is very insoluble in water, but after oxidation it can be converted to uranium trioxide or another uranium(VI) compound which is much more soluble. Uranium dioxide (UO2) can be oxidised to an oxygen rich hyperstoichiometric oxide (UO2+x) which can be further oxidised to U4O9, U3O7, U3O8 and UO3.2H2O.
Because used fuel contains alpha emitters (plutonium and the minor actinides), the effect of adding an alpha emitter (238Pu) to uranium dioxide on the leaching rate of the oxide has been investigated. For the crushed oxide, adding 238Pu tended to increase the rate of leaching, but the difference in the leaching rate between 0.1 and 10% 238Pu was very small.[13]
The concentration of carbonate in the water which is in contact with the used fuel has a considerable effect on the rate of corrosion, because uranium(VI) forms soluble anionic carbonate complexes such as [UO2(CO3)2]2- and [UO2(CO3)3]4-. When carbonate ions are absent, and the water is not strongly acidic, the hexavalent uranium compounds which form on oxidation of uranium dioxide often form insoluble hydrated uranium trioxide phases.[14]
By ‘sputtering’, using uranium metal and an argon/oxygen gas mixture, thin films of uranium dioxide can be deposited upon gold surfaces. These gold surfaces modified with uranium dioxide have been used for both cyclic voltammetry and AC impedance experiments, and these offer an insight into the likely leaching behaviour of uranium dioxide.[15]
Fuel cladding interactions
The study of the nuclear fuel cycle includes the study of the behaviour of nuclear materials both under normal conditions and under accident conditions. For example, there has been much work on how uranium dioxide based fuel interacts with the zirconium alloy tubing used to cover it. During use, the fuel swells due to thermal expansion and then starts to react with the surface of the zirconium alloy, forming a new layer which contains both fuel and zirconium (from the cladding). Then, on the fuel side of this mixed layer, there is a layer of fuel which has a higher caesium to uranium ratio than most of the fuel. This is because xenon isotopes are formed as fission products that diffuse out of the lattice of the fuel into voids such as the narrow gap between the fuel and the cladding. After diffusing into these voids, it decays to caesium isotopes. Because of the thermal gradient which exists in the fuel during use, the volatile fission products tend to be driven from the centre of the pellet to the rim area.[16] Below is a graph of the temperature of uranium metal, uranium nitride and uranium dioxide as a function of distance from the centre of a 20 mm diameter pellet with a rim temperature of 200 oC. The uranium dioxide (because of its poor thermal conductivity) will overheat at the centre of the pellet, while the other more thermally conductive forms of uranium remain below their melting points.
Normal and abnormal conditions
The nuclear chemistry associated with the nuclear fuel cycle can be divided into two main areas, one area is concerned with operation under the intended conditions while the other area is concerned with maloperation conditions where some alteration from the normal operating conditions has occurred or (more rarely) an accident is occurring.
The releases of radioactivity from normal operations are the small planned releases from uranium ore processing, enrichment, power reactors, reprocessing plants and waste stores. These can be in a different chemical/physical form to the releases which could occur under accident conditions. In addition the isotope signature of a hypothetical accident may be very different to that of a planned normal operational discharge of radioactivity to the environment.
Just because a radioisotope is released it does not mean it will enter a human and then cause harm. For instance the migration of radioactivity can be altered by the binding of the radioisotope to the surfaces of soil particles. For example caesium binds tightly to clay minerals such as illite and montmorillonite hence it remains in the upper layers of soil where it can be accessed by plants with shallow roots (such as grass). Hence grass and mushrooms can carry a considerable amount of 137Cs which can be transferred to humans through the food chain. But 137Cs is not able to migrate quickly through most soils and thus is unlikely to contaminate well water. Colloids of soil minerals can migrate through soil so simple binding of a metal to the surfaces of soil particles does not fix the metal totally.
According to Jiří Hála's text book the distribution coefficient Kd is the ratio of the soil's radioactivity (Bq g−1) to that of the soil water (Bq ml−1). If the radioisotope is tightly bound to the minerals in the soil then less radioactivity can be absorbed by crops and grass growing on the soil.
One of the best countermeasures in dairy farming against 137Cs is to mix up the soil by deeply ploughing the soil. This has the effect of putting the 137Cs out of reach of the shallow roots of the grass, hence the level of radioactivity in the grass will be lowered. Also after a nuclear war or serious accident the removal of top few cm of soil and its burial in a shallow trench will reduce the long term gamma dose to humans due to 137Cs as the gamma photons will be attenuated by their passage through the soil.
Even after the radioactive element arrives at the roots of the plant, the metal may be rejected by the biochemistry of the plant. The details of the uptake of 90Sr and 137Cs into sunflowers grown under hydroponic conditions has been reported.[17] The caesium was found in the leaf veins, in the stem and in the apical leaves. It was found that 12% of the caesium entered the plant, and 20% of the strontium. This paper also reports details of the effect of potassium, ammonium and calcium ions on the uptake of the radioisotopes.
In livestock farming an important countermeasure against 137Cs is to feed to animals a little prussian blue. This iron potassium cyanide compound acts as a ion-exchanger. The cyanide is so tightly bonded to the iron that it is safe for a human to eat several grams of prussian blue per day. The prussian blue reduces the biological half life (different from the nuclear half life) of the caesium. The physical or nuclear half life of 137Cs is about 30 years. This is a constant which can not be changed but the biological half life is not a constant. It will change according to the nature and habits of the organism for which it is expressed. Caesium in humans normally has a biological half life of between one and four months. An added advantage of the prussian blue is that the caesium which is stripped from the animal in the droppings is in a form which is not available to plants. Hence it prevents the caesium from being recycled. The form of prussian blue required for the treatment of humans or animals is a special grade. Attempts to use the pigment grade used in paints have not been successful. Note that a good source of data on the subject of caesium in Chernobyl fallout exists at [1], this is the Ukrainian Research Institute for Agricultural Radiology.
Release of radioactivity from fuel during normal use and accidents
The IAEA assume that under normal operation the coolant of a water cooled reactor will contain some radioactivity[18] but during a reactor accident the coolant radioactivity level may rise. The IAEA state that under a series of different conditions different amounts of the core inventory can be released from the fuel, the four conditions the IAEA consider are normal operation, a spike in coolant activity due to a sudden shutdown/loss of pressure (core remains covered with water), a cladding failure resulting in the release of the activity in the fuel/cladding gap (this could be due to the fuel being uncovered by the loss of water for 15–30 minutes where the cladding reached a temperature of 650-1250 oC) or a melting of the core (the fuel will have to be uncovered for at least 30 minutes, and the cladding would reach a temperature in excess of 1650 oC).[19]
Based upon the assumption that a PWR contains 300 tons of water, and that the activity of the fuel of a 1 GWe reactor is as the IAEA predict,[20] then the coolant activity after an accident such as the three mile island accident where a core is uncovered and then recovered with water then the resulting activity of the coolant can be predicted.
Releases from reprocessing under normal conditions
It is normal to allow used fuel to stand after the irradiation to allow the short-lived and radiotoxic iodine isotopes to decay away, in one experiment in the USA fresh fuel which had not been allowed to decay was reprocessed (the Green run[2][3][4]) to investigate the effects of a large iodine release from the reprocessing of short cooled fuel. It is normal in reprocessing plants to scrub the off gases from the dissolver to prevent the emission of iodine. In addition to the emission of iodine the noble gases and tritium are released from the fuel when it is dissolved, it has been proposed that by voloxidation (heating the fuel in a furnace under oxidizing conditions) the majority of the tritium can be recovered from the fuel.[5]
A paper was written on the radioactivity found in oysters found in the Irish Sea,[21] these were found by gamma spectrscopy to contain 141Ce, 144Ce, 103Ru, 106Ru, 137Cs, 95Zr and 95Nb. In addition a zinc activation product (65Zn) was found, this is thought to be due to the corrosion of magnox fuel cladding in cooling ponds. It is likely that the modern releases of all these isotopes from Windscale is smaller.
On-load reactors
Some reactor designs, such as RBMKs or CANDU reactors, can be refueled without being shut down. This is achieved through the use of many small pressure tubes to contain the fuel and coolant, as opposed to one large pressure vessel as in pressurized water reactor (PWR) or boiling water reactor (BWR) designs. Each tube can be individually isolated and refueled by an operator-controlled fueling machine, typically at a rate of up to 8 channels per day out of roughly 400 in CANDU reactors. On-load refueling allows for the problem of optimal fuel reloading problem to be dealt with continuously, leading to more efficient use of fuel. This increase in efficiency is partially offset by the added complexity of having hundreds of pressure tubes and the fueling machines to service them.
Back end
Interim storage
After its operating cycle, the reactor is shut down for refueling. The fuel discharged at that time (spent fuel) is stored either at the reactor site, commonly in a spent fuel pool or, potentially in a common facility away from reactor sites. If on-site pool storage capacity is exceeded, it may be desirable to store the now cooled aged fuel in modular dry storage facilities known as Independent Spent Fuel Storage Installations (ISFSI) at the reactor site or at a facility away from the site. The spent fuel rods are usually stored in water or boric acid, which provides both cooling, the spent fuel continues to generate decay heat as a result of residual radioactive decay, and shielding to protect the environment from residual ionizing radiation, although after several years of cooling they may be moved to dry cask storage.
Transportation
Reprocessing
Spent fuel discharged from reactors contains appreciable quantities of fissile (U-235 and Pu-239), fertile (U-238), and other radioactive materials, including reaction poisons, which is why the fuel had to be removed. These fissile and fertile materials can be chemically separated and recovered from the spent fuel. The recovered uranium and plutonium can, if economic and institutional conditions permit, be recycled for use as nuclear fuel. This is currently not done for civilian spent nuclear fuel in the US.
Mixed oxide, or MOX fuel, is a blend of reprocessed uranium and plutonium and depleted uranium which behaves similarly, although not identically, to the enriched uranium feed for which most nuclear reactors were designed. MOX fuel is an alternative to low-enriched uranium (LEU) fuel used in the light water reactors which predominate nuclear power generation.
Currently, plants in Europe are reprocessing spent fuel from utilities in Europe and Japan. Reprocessing of spent commercial-reactor nuclear fuel is currently not permitted in the United States due to the perceived danger of nuclear proliferation. However the recently announced Global Nuclear Energy Partnership would see the U.S. form an international partnership to see spent nuclear fuel reprocessed in a way that renders the plutonium in it usable for nuclear fuel but not for nuclear weapons.
Partitioning and transmutation
As an alternative to the disposal of the PUREX raffinate in glass or Synroc, the most radiotoxic elements can be removed through advanced reprocessing. After separation the minor actinides and some long lived fission products can be converted to short-lived isotopes by either neutron or photon irradiation. This is called transmutation.
Waste disposal
A current concern in the nuclear power field is the safe disposal and isolation of either spent fuel from reactors or, if the reprocessing option is used, wastes from reprocessing plants. These materials must be isolated from the biosphere until the radioactivity contained in them has diminished to a safe level.[22] In the U.S., under the Nuclear Waste Policy Act of 1982 as amended, the Department of Energy has responsibility for the development of the waste disposal system for spent nuclear fuel and high-level radioactive waste. Current plans call for the ultimate disposal of the wastes in solid form in a licensed deep, stable geologic structure called a deep geological repository. The Department of Energy chose Yucca Mountain as the location for the repository. However, its opening has been repeatedly delayed.
It is worth noting that some non-PLWR reactor designs, and in particular the ones using liquid thorium fuel in molten salt reactors, would produce virtually no long-lasting nuclear waste. It is also possible to burn rather than bury nuclear waste for instance in Integral Fast Reactor or in variation of molten salt reactor.[citation needed]
A proposed type of nuclear reactor called a traveling wave reactor is claimed, if it were to be built, to be able to be fueled by nuclear waste, and to be able to operate for 200 years without needing any refueling.[23]
Integrated Nuclear Fuel Cycle Information System
Integrated nuclear fuel cycle information system (iNFCIS) is a set of databases related to the nuclear fuel cycle maintained by the International Atomic Energy Agency (IAEA). iNFCIS provides information on various aspects of nuclear fuel cycle. Presently iNFCIS includes UDEPO - World distribution of uranium deposits; NFCIS - Nuclear fuel cycle information system, a database of civilian nuclear fuel cycle facilities; PIEDB - Post irradiation examination facilities database; MABD - Minor actinide property database and NFCSS - Nuclear fuel cycle simulation system, a tool for modeling material flow and actinide accumulations in the nuclear fuel cycle. iNFCIS requires free registration for on-line access.
See also
- Synthesis of noble metals
- Deep geological repository
- Nuclear reprocessing
- Enrico Fermi
- Global Nuclear Energy Partnership announced February, 2006
- Manhattan Project
- Nuclear physics
- Nuclear power debate
- Uranium mining debate
- United States Naval reactor
References
- ^ "Management of Spent Fuel at Nuclear Power Plants". IAEA Bulletin. Retrieved 2008-01-15.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "The Preparation of the EFTTRA-T5 Americium Transmutation Experiment" (PDF). Seventh Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation. 2002. Retrieved 2008-01-15.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ Gudowski, W. (2000). "Why Accelerator-Driven Transmutation of Wastes Enables Future Nuclear Power?" (PDF). XX International Linac Conference. Retrieved 2008-01-15.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Heighway, E. A. (1994-08-01). "An overview of accelerator-driven transmutation technology" (PDF). Retrieved 2008-01-15.
- ^ "Accelerator-driven Systems (ADS) and Fast Reactors (FR) in Advanced Nuclear Fuel Cycles" (PDF). Nuclear Energy Agency. Retrieved 2008-01-15.
- ^ "Concept of a Small-scale Electron Accelerator Driven System for Nuclear Waste Transmutation Part 2. Investigation of burnup" (PDF). ScienceDirect. 2005. Retrieved 2008-01-15.
{{cite web}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - ^ a b See thorium fuel cycle
- ^ See Thorium occurrence for discussion of abundance.
- ^ Dr. Chidambaram R. (1997). "Towards an Energy Independent India". Nu-Power. Nuclear Power Corporation of India Limited. Archived from the original on 2007-12-17. Retrieved 2008-01-15.
- ^ "How much depleted uranium hexafluoride is stored in the United States?". Depleted UF6 Management Information Network. Retrieved 2008-01-15.
- ^ "Susquehanna Nuclear Energy Guide" (PDF). PPL Corporation. Retrieved 2008-01-15.
- ^ A good report on the microstructure of used fuel is Lucuta PG et al. (1991) J Nuclear Materials 178:48-60
- ^ V.V. Rondinella VV et al. (2000) Radiochimica Acta 88:527-531
- ^ For a review of the corrosion of uranium dioxide in a waste store which explains much of the chemistry, see Shoesmith DW (2000) J Nuclear Materials 282:1-31
- ^ Miserque F et al. (2001) J Nuclear Materials 298:280-90
- ^ Further reading on fuel cladding interactions: Tanaka K et al. (2006) J Nuclear Materials 357:58-68
- ^ P. Soudek, Š. Valenová, Z. Vavříková and T. Vaněk, Journal of Environmental Radioactivity, 2006, 88, 236-250
- ^ page 169 Generic Assessment Procedures for Determining Protective Actions During a Reactor Accident, IAEA-TECDOC-955, 1997
- ^ page 173 Generic Assessment Procedures for Determining Protective Actions During a Reactor Accident, IAEA-TECDOC-955, 1997
- ^ page 171 Generic Assessment Procedures for Determining Protective Actions During a Reactor Accident, IAEA-TECDOC-955, 1997
- ^ A. Preston, J.W.R. Dutton and B.R. Harvey, Nature, 1968, 218, 689-690.
- ^ M. I. Ojovan, W.E. Lee. An Introduction to Nuclear Waste Immobilisation, Elsevier Science Publishers B.V., ISBN 0-08-044462-8, Amsterdam, 315pp. (2005).
- ^ TR10: Traveling Wave Reactor, Technology Review, March/April 2009
(Reference V. Artisyuk, M. Saito and A. Shmelev, Progress in Nuclear Energy, 2000, 37, 345-350)
External links
- IAEA Integrated Nuclear Fuel Cycle Information System - (Free registration required)
- BBC - The Nuclear Fuel Cycle
- Chalmers University of Technology - In-Core Fuel Management (PDF)
- HYKE - Assembly Distribution Optimality Condition (PS)
- Nuclear Files - The Nuclear Fuel Cycle
- WISE - Nuclear Fuel Energy Balance Calculator
- World Nuclear Association - Reprocessing Facilities
- Annotated bibliography on the nuclear fuel cycle from the Alsos Digital Library for Nuclear Issues
- Documents Related to Liquid-Halide (Fluoride and Chloride) Reactor Research and Development Textbooks, journal articles, ORNL reports