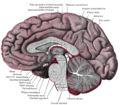
Pineal gland
| Pineal gland | |
|---|---|
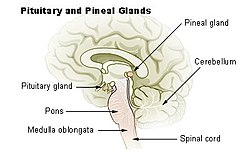 Diagram of pituitary and pineal glands in the human brain | |
| Details | |
| Precursor | Neural ectoderm, roof of diencephalon |
| Artery | posterior cerebral artery |
| Identifiers | |
| Latin | glandula pinealis |
| MeSH | D010870 |
| NeuroNames | 297 |
| NeuroLex ID | birnlex_1184 |
| TA98 | A11.2.00.001 |
| TA2 | 3862 |
| FMA | 62033 |
| Anatomical terms of neuroanatomy | |

The pineal gland, also known as the conarium or epiphysis cerebri, is a small endocrine gland in the vertebrate brain. The pineal gland produces melatonin, a serotonin-derived hormone which modulates sleep patterns in both circadian and seasonal cycles. The shape of the gland resembles a pine cone, hence its name. The pineal gland is located in the epithalamus, near the center of the brain, between the two hemispheres, tucked in a groove where the two halves of the thalamus join.[1][2]
Nearly all vertebrate species possess a pineal gland. The most important exception is a primitive vertebrate, the hagfish. Even in the hagfish, however, there may be a "pineal equivalent" structure in the dorsal diencephalon.[3] The lancelet Branchiostoma lanceolatum, the nearest existing relative to vertebrates, also lacks a recognizable pineal gland.[4] The lamprey (another primitive vertebrate), however, does possess one.[4] A few more developed vertebrates lost pineal glands over the course of their evolution.[5]
The results of various scientific research in evolutionary biology, comparative neuroanatomy and neurophysiology, have explained the phylogeny of the pineal gland in different vertebrate species. From the point of view of biological evolution, the pineal gland represents a kind of atrophied photoreceptor. In the epithalamus of some species of amphibians and reptiles, it is linked to a light-sensing organ, known as the parietal eye, which is also called the pineal eye or third eye.[6]
René Descartes believed the pineal gland to be the "principal seat of the soul". Academic philosophy among his contemporaries considered the pineal gland as a neuroanatomical structure without special metaphysical qualities; science studied it as one endocrine gland among many. However, the pineal gland continues to have an exalted status in the realm of pseudoscience.[7]
Structure
The pineal gland is a midline brain structure that is unpaired. It takes its name from its pine-cone shape.[8] The gland is reddish-gray and about the size of a grain of rice (5–8 mm) in humans. The pineal gland, also called the pineal body, is part of the epithalamus, and lies between the laterally positioned thalamic bodies and behind the habenular commissure. It is located in the quadrigeminal cistern near to the corpora quadrigemina.[9] It is also located behind the third ventricle and is bathed in cerebrospinal fluid supplied through a small pineal recess of the third ventricle which projects into the stalk of the gland.[10]
Blood supply
Unlike most of the mammalian brain, the pineal gland is not isolated from the body by the blood–brain barrier system;[11] it has profuse blood flow, second only to the kidney,[12] supplied from the choroidal branches of the posterior cerebral artery.
Nerve supply
The pineal gland receives a sympathetic innervation from the superior cervical ganglion. A parasympathetic innervation from the pterygopalatine and otic ganglia is also present.[13] Further, some nerve fibers penetrate into the pineal gland via the pineal stalk (central innervation). Also, neurons in the trigeminal ganglion innervate the gland with nerve fibers containing the neuropeptide PACAP.
Microanatomy
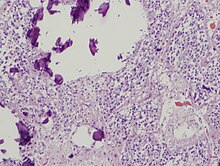
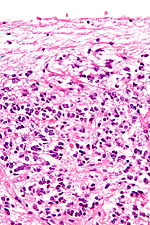
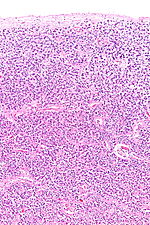
The pineal body consists in humans of a lobular parenchyma of pinealocytes surrounded by connective tissue spaces. The gland's surface is covered by a pial capsule.
The pineal gland consists mainly of pinealocytes, but four other cell types have been identified. As it is quite cellular (in relation to the cortex and white matter), it may be mistaken for a neoplasm.[14]
| Cell type | Description |
|---|---|
| Pinealocytes | The pinealocytes consist of a cell body with 4–6 processes emerging. They produce and secrete melatonin. The pinealocytes can be stained by special silver impregnation methods. Their cytoplasm is lightly basophilic. With special stains, pinealocytes exhibit lengthy, branched cytoplasmic processes that extend to the connective septa and its blood vessels. |
| Interstitial cells | Interstitial cells are located between the pinealocytes. They have elongated nuclei and a cytoplasm that is stained darker than that of the pinealocytes. |
| Perivascular phagocyte | Many capillaries are present in the gland, and perivascular phagocytes are located close to these blood vessels. The perivascular phagocytes are antigen presenting cells. |
| Pineal neurons | In higher vertebrates neurons are usually located in the pineal gland. However, this is not the case in rodents. |
| Peptidergic neuron-like cells | In some species, neuronal-like peptidergic cells are present. These cells might have a paracrine regulatory function. |
In some parts of the brain and in particular the pineal gland, there are calcium structures, the number of which increases with age, called corpora arenacea (or "acervuli," or "brain sand"). Chemical analysis shows that they are composed of calcium phosphate, calcium carbonate, magnesium phosphate, and ammonium phosphate.[15] In 2002, deposits of the calcite form of calcium carbonate were described.[16] Calcium and phosphorus[17] deposits in the pineal gland have been linked with aging.
Development
The human pineal gland grows in size until about 1–2 years of age, remaining stable thereafter,[18][19] although its weight increases gradually from puberty onwards.[20][21] The abundant melatonin levels in children are believed to inhibit sexual development, and pineal tumors have been linked with precocious puberty. When puberty arrives, melatonin production is reduced.[citation needed]
Symmetry
In the zebrafish the pineal gland does not straddle the midline but shows a left-sided bias. In humans, functional cerebral dominance is accompanied by subtle anatomical asymmetry.[22][23][24]
Function
The primary function of the pineal gland is to produce melatonin. Melatonin has various functions in the central nervous system, the most important of which is to help modulate sleep patterns. Melatonin production is stimulated by darkness and inhibited by light.[25][26] Light sensitive nerve cells in the retina detect light and send this signal to the suprachiasmatic nucleus (SCN), synchronizing the SCN to the day-night cycle. Nerve fibers then relay the daylight information from the SCN to the paraventricular nuclei (PVN), then to the spinal cord and via the sympathetic system to superior cervical ganglia (SCG), and from there into the pineal gland.
The compound pinoline is also claimed to be produced in the pineal gland; it is one of the beta-carbolines.[27] This claim is subject to some controversy.
Regulation of the pituitary gland
Studies on rodents suggest that the pineal gland influences the pituitary gland's secretion of the sex hormones, follicle-stimulating hormone (FSH), and luteinizing hormone (LH). Pinealectomy performed on rodents produced no change in pituitary weight, but caused an increase in the concentration of FSH and LH within the gland.[28] Administration of melatonin did not return the concentrations of FSH to normal levels, suggesting that the pineal gland influences pituitary gland secretion of FSH and LH through an undescribed transmitting molecule.[28]
The pineal gland contains receptors for the regulatory neuropeptide, endothelin-1,[29] which, when injected in picomolar quantities into the lateral cerebral ventricle, causes a calcium-mediated increase in pineal glucose metabolism.[30]
Drug metabolism
Studies on rodents suggest that the pineal gland may influence the actions of recreational drugs, such as cocaine,[31] and antidepressants, such as fluoxetine (Prozac),[32] and that its hormone melatonin can protect against neurodegeneration.[33]
Regulation of bone metabolism
Studies in mice suggest that the pineal-derived melatonin regulates new bone deposition. Pineal-derived melatonin mediates its action on the bone cells through MT2 receptors. This pathway could be a potential new target for osteoporosis treatment as the study shows the curative effect of oral melatonin treatment in a postmenopausal osteoporosis mouse model.[34]
Clinical significance
Calcification
Calcification of the pineal gland is typical in young adults, and has been observed in children as young as two years of age.[35] Calcium and phosphorus deposits in the pineal gland have been correlated with aging.[17] By old age, the pineal gland contains about the same amount of fluoride as teeth.[36] Pineal fluoride and pineal calcium are correlated.[36]
The calcified gland is often seen in skull X-Rays.[35] Calcification rates vary widely by country and correlate with an increase in age, with calcification occurring in an estimated 40% of Americans by age seventeen.[35] Calcification of the pineal gland is associated with corpora arenacea, also known as "brain sand".
It seems that the internal secretions of the pineal gland inhibit the development of the reproductive glands because when it is severely damaged in children, development of the sexual organs and the skeleton are accelerated.[37]
Some studies show that the degree of pineal gland calcification is significantly higher in patients with Alzheimer's disease vs. other types of dementia.[38] Pineal gland calcification may contribute to the pathogenesis of Alzheimer's disease and may reflect an absence of crystallization inhibitors.[38] Calcification of the pineal gland has also been found to be closely associated to certain types of migraines as well as cluster headaches.[39][36]
Tumours
Tumours of the pineal gland are called pinealomas. These tumours are rare and 50% to 70% are germinomas that arise from sequestered embryonic germ cells. Histologically they are similar to testicular seminomas and ovarian dysgerminomas.[40]
A pineal tumour can compress the superior colliculi and pretectal area of the dorsal midbrain, producing Parinaud's syndrome. Pineal tumours also can cause compression of the cerebral aqueduct, resulting in a noncommunicating hydrocephalus. Other manifestations are the consequence of their pressure effects and consist of visual disturbances, headache, mental deterioration, and sometimes dementia-like behaviour.[41]
These neoplasms are divided into three categories, pineoblastomas, pineocytomas, and mixed tumours, based on their level of differentiation, which, in turn, correlates with their neoplastic aggressiveness.[42] The clinical course of patients with pineocytomas is prolonged, averaging up to several years.[43] The position of these tumours makes them very difficult to remove surgically.
Other animals
Most living vertebrates have pineal glands. It's likely that the common ancestor of all vertebrates had a pair of photosensory organs on the top of its head, similar to the arrangement in modern lampreys.[44] Some extinct Devonian fishes have two parietal foramina in their skulls,[45][46] suggesting an ancestral bilaterality of parietal eyes. The parietal eye and the pineal gland of living tetrapods are probably the descendants of the left and right parts of this organ, respectively.[47]
During embryonic development, the parietal eye and the pineal organ of modern lizards[48] and tuataras[49] form together from a pocket formed in the brain ectoderm. The loss of parietal eyes in many living tetrapods is supported by developmental formation of a paired structure that subsequently fuses into a single pineal gland in developing embryos of turtles, snakes, birds, and mammals.[50]
The pineal organs of mammals fall into one of three categories based on shape. Rodents have more structurally-complex pineal glands than other mammals.[51]
Crocodilians and some tropical lineages of mammals (some xenarthrans [sloths], pangolins, sirenians [manatees & dugongs], and some marsupials [sugar gliders]) have lost both their parietal eye and their pineal organ.[52][53][51] Polar mammals, such as walruses and some seals, possess unusually large pineal glands.[52]
All amphibians have a pineal organ, but some frogs and toads also have what is called a "frontal organ", which is essentially a parietal eye.[54]
Pinealocytes in many non-mammalian vertebrates have a strong resemblance to the photoreceptor cells of the eye. Evidence from morphology and developmental biology suggests that pineal cells possess a common evolutionary ancestor with retinal cells.[55]
Pineal cytostructure seems to have evolutionary similarities to the retinal cells of the lateral eyes.[55] Modern birds and reptiles express the phototransducing pigment melanopsin in the pineal gland. Avian pineal glands are thought to act like the suprachiasmatic nucleus in mammals.[56] The structure of the pineal eye in modern lizards and tuatara is analogous to the cornea, lens, and retina of the lateral eyes of vertebrates.[50]
In most vertebrates, exposure to light sets off a chain reaction of enzymatic events within the pineal gland that regulates circadian rhythms.[57] In humans and other mammals, the light signals necessary to set circadian rhythms are sent from the eye through the retinohypothalamic system to the suprachiasmatic nuclei (SCN) and the pineal gland.
The fossilized skulls of many extinct vertebrates have a pineal foramen (opening), which in some cases is larger than that of any living vertebrate.[58] Although fossils seldom preserve deep-brain soft anatomy, the brain of the Russian fossil bird Cerebavis cenomanica from Melovatka, about 90 million years old, shows a relatively large parietal eye and pineal gland.[59]
Society and culture
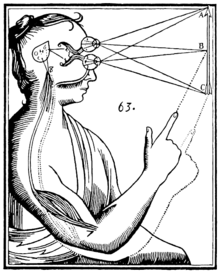
Seventeenth-century philosopher and scientist René Descartes was highly interested in anatomy and physiology. He discussed the pineal gland both in his first book, the Treatise of Man (written before 1637, but only published posthumously 1662/1664), and in his last book, The Passions of the Soul (1649) and he regarded it as "the principal seat of the soul and the place in which all our thoughts are formed."[7] In the Treatise of Man, Descartes described conceptual models of man, namely creatures created by God, which consist of two ingredients, a body and a soul.[7][60] In the Passions, Descartes split man up into a body and a soul and emphasized that the soul is joined to the whole body by "a certain very small gland situated in the middle of the brain's substance and suspended above the passage through which the spirits in the brain's anterior cavities communicate with those in its posterior cavities". Descartes attached significance to the gland because he believed it to be the only section of the brain to exist as a single part rather than one-half of a pair. Most of Descartes's basic anatomical and physiological assumptions were totally mistaken, not only by modern standards, but also in light of what was already known in his time.[7][61]
The notion of a "pineal-eye" is central to the philosophy of the French writer Georges Bataille, which is analyzed at length by literary scholar Denis Hollier in his study Against Architecture. In this work Hollier discusses how Bataille uses the concept of a "pineal-eye" as a reference to a blind-spot in Western rationality, and an organ of excess and delirium.[62] This conceptual device is explicit in his surrealist texts, The Jesuve and The Pineal Eye.[63]
In the late 19th century Madame Blavatsky (who founded theosophy) identified the pineal gland with the Hindu concept of the third eye, or the Ajna chakra. This association is still popular today.[7]
Rick Strassman, an author and Clinical Associate Professor of Psychiatry at the University of New Mexico School of Medicine, has theorised that the human pineal gland is capable of producing the hallucinogen N,N-dimethyltryptamine (DMT) under certain circumstances.[64] In 2013 he and other researchers first reported DMT in the pineal gland microdialysate of rodents.[65]
In the short story "From Beyond" by H. P. Lovecraft, a scientist creates an electronic device that emits a resonance wave, which stimulates an affected person's pineal gland, thereby allowing her or him to perceive planes of existence outside the scope of accepted reality, a translucent, alien environment that overlaps our own recognized reality. It was adapted as a film of the same name in 1986. The 2013 horror film, Banshee Chapter is heavily influenced by this short story.
History
The secretory activity of the pineal gland is only partially understood. Its location deep in the brain suggested to philosophers throughout history that it possesses particular importance. This combination led to its being regarded as a "mystery" gland with mystical, metaphysical, and occult theories surrounding its perceived functions.
The pineal gland was originally believed to be a "vestigial remnant" of a larger organ. In 1917, it was known that extract of cow pineals lightened frog skin. Dermatology professor Aaron B. Lerner and colleagues at Yale University, hoping that a substance from the pineal might be useful in treating skin diseases, isolated and named the hormone melatonin in 1958.[66] The substance did not prove to be helpful as intended, but its discovery helped solve several mysteries such as why removing the rat's pineal accelerated ovary growth, why keeping rats in constant light decreased the weight of their pineals, and why pinealectomy and constant light affect ovary growth to an equal extent; this knowledge gave a boost to the then new field of chronobiology.[67]
See also
Additional images
The pineal body is labeled in these images.
-
Mesal aspect of a brain sectioned in the median sagittal plane.
-
Dissection showing the ventricles of the brain.
-
Hind- and mid-brains; antero-lateral view.
-
Median sagittal section of brain.
-
Pineal gland
-
Brainstem. Posterior view.
References
- ^ Macchi M, Bruce J (2004). "Human pineal physiology and functional significance of melatonin". Front Neuroendocrinol. 25 (3–4): 177–95. doi:10.1016/j.yfrne.2004.08.001. PMID 15589268.
- ^ Arendt J, Skene DJ (2005). "Melatonin as a chronobiotic". Sleep Med Rev. 9 (1): 25–39. doi:10.1016/j.smrv.2004.05.002. PMID 15649736.
Exogenous melatonin has acute sleepiness-inducing and temperature-lowering effects during 'biological daytime', and when suitably timed (it is most effective around dusk and dawn) it will shift the phase of the human circadian clock (sleep, endogenous melatonin, core body temperature, cortisol) to earlier (advance phase shift) or later (delay phase shift) times.
- ^ Ooka-Souda S, Kadota T, Kabasawa H (December 1993). "The preoptic nucleus: the probable location of the circadian pacemaker of the hagfish, Eptatretus burgeri". Neurosci. Lett. 164 (1–2): 33–6. doi:10.1016/0304-3940(93)90850-K. PMID 8152610.
- ^ a b Vernadakis AJ, Bemis WE, Bittman EL (April 1998). "Localization and partial characterization of melatonin receptors in amphioxus, hagfish, lamprey, and skate". Gen. Comp. Endocrinol. 110 (1): 67–78. doi:10.1006/gcen.1997.7042. PMID 9514841.
- ^ Erlich SS, Apuzzo ML (September 1985). "The pineal gland: anatomy, physiology, and clinical significance". J. Neurosurg. 63 (3): 321–41. doi:10.3171/jns.1985.63.3.0321. PMID 2862230.
- ^ Eakin, Richard M. (1973). The Third Eye. Berkeley: University of California Press.
- ^ a b c d e Lokhorst, Gert-Jan (2015). Descartes and the Pineal Gland. Stanford: The Stanford Encyclopedia of Philosophy.
- ^ Bowen, R. "The Pineal Gland and Melatonin". Archived from the original on 24 November 2011. Retrieved 14 October 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ http://www.ajnr.org/content/19/9/1631.full.pdf
- ^ Dorland's. Illustrated Medical Dictionary. Elsevier Saunders. p. 1607. ISBN 978-1-4160-6257-8.
- ^ Pritchard, Thomas C.; Alloway, Kevin Douglas (1999). Medical Neuroscience (Google books preview). Hayes Barton Press. pp. 76–77. ISBN 1-889325-29-5. Retrieved 8 February 2009.
- ^ Arendt J: Melatonin and the Mammalian Pineal Gland, ed 1. London. Chapman & Hall, 1995, p 17
- ^ Møller, M., Baeres, F.M.M. (2014). "The anatomy and innervation of the mammalian pineal gland". Cell Tissue Res. 309 (1): 139–150. doi:10.1007/s00441-002-0580-5. PMID 12111544.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kleinschmidt-DeMasters BK, Prayson RA (November 2006). "An algorithmic approach to the brain biopsy—part I". Arch. Pathol. Lab. Med. 130 (11): 1630–8. doi:10.1043/1543-2165(2006)130[1630:AAATTB]2.0.CO;2 (inactive 21 August 2017). PMID 17076524.
{{cite journal}}: CS1 maint: DOI inactive as of August 2017 (link) - ^ Bocchi G, Valdre G (1993). "Physical, chemical, and mineralogical characterization of carbonate-hydroxyapatite concretions of the human pineal gland". J Inorg Biochem. 49 (3): 209–20. doi:10.1016/0162-0134(93)80006-U. PMID 8381851.
- ^ Baconnier S, Lang S, Polomska M, Hilczer B, Berkovic G, Meshulam G (2002). "Calcite microcrystals in the pineal gland of the human brain: first physical and chemical studies". Bioelectromagnetics. 23 (7): 488–95. doi:10.1002/bem.10053. PMID 12224052.
- ^ a b "IngentaConnect High Accumulation of Calcium and Phosphorus in the Pineal Bodies". Ingentaconnect.com. 16 June 2006. Archived from the original on 21 October 2012. Retrieved 6 July 2009.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Schmidt, F; Penka, B; Trauner, M; Reinsperger, L; Ranner, G; Ebner, F; Waldhauser, F (April 1995). "Lack of pineal growth during childhood". J Clin Endocrinol Metab. 80 (4): 1221–5. doi:10.1210/JCEM.80.4.7536203. PMID 7536203.
- ^ Sumida, M; Barkovich, AJ; Newton, TH (February 1996). "Development of the pineal gland: measurement with MR". AJNR Am J Neuroradiol. 17 (2): 233–6. PMID 8938291.
- ^ Tapp, E; Huxley, M (1971). "The weight and degree of calcification of the pineal gland". J Pathol. 105 (1): 31–39. doi:10.1002/path.1711050105. PMID 4943068.
- ^ Tapp, E; Huxley, M (1972). "The histological appearance of the human pineal gland from puberty to old age". J Pathol. 108 (2): 137–144. doi:10.1002/path.1711080207. PMID 4647506.
- ^ Snelson, CD; Santhakumar, K; Halpern, ME; Gamse, JT (May 2008). "Tbx2b is required for the development of the parapineal organ". Development. 135 (9): 1693–702. doi:10.1242/dev.016576. PMC 2810831. PMID 18385257.
- ^ Snelson, CD; Burkart, JT; Gamse, JT (December 2008). "Formation of the asymmetric pineal complex in zebrafish requires two independently acting transcription factors". Developmental Dynamics. 237 (12): 3538–44. doi:10.1002/dvdy.21607. PMC 2810829. PMID 18629869.
- ^ Snelson, Corey D.; Gamse, Joshua T. (June 2009). "Building an asymmetric brain: Development of the zebrafish epithalamus". Seminars in Cell & Developmental Biology. 20 (4): 491–497. doi:10.1016/j.semcdb.2008.11.008. PMC 2729063. PMID 19084075.
- ^ Axelrod J (1970). "The pineal gland". Endeavour. 29 (108): 144–8. PMID 4195878.
- ^ Lowrey, Phillip L.; Takahashi, Joseph S. (2000). "Genetics of the Mammalian Circadian System: Photic Entrainment, Circadian Pacemaker Mechanisms, and Posttranslational Regulation". Annual Review of Genetics. 34 (1): 533–562. doi:10.1146/annurev.genet.34.1.533.
- ^ Callaway, James C.; Gyntber, Jukka; Poso, Antti; Airaksinen, Mauno M.; Vepsäläinen, Jouko (1994). "The pictet-spengler reaction and biogenic tryptamines: Formation of tetrahydro-β-carbolines at physiologicalpH". Journal of Heterocyclic Chemistry. 31 (2): 431–435. doi:10.1002/jhet.5570310231.
- ^ a b Motta, Marina; Fraschini, F.; Martini, L. (1967). "Endocrine Effects of Pineal Gland and of Melatonin". Exp Biol Med (Maywood). 126 (2): 431–435. doi:10.3181/00379727-126-32468.
- ^ Naidoo, V; Naidoo, S; Mahabeer, R; Raidoo, DM (2004). "Cellular distribution of the endothelin system in the human brain". Journal of Chemical Neuroanatomy. 27 (2): 87–98. doi:10.1016/j.jchemneu.2003.12.002. PMID 15121213.
- ^ Gross, PM; Wainman, DS; Chew, BH; Espinosa, FJ; Weaver, DF (1993). "Calcium-mediated metabolic stimulation of neuroendocrine structures by intraventricular endothelin-1 in conscious rats". Brain Research. 606 (1): 135–42. PMID 8461995.
- ^ Uz T, Akhisaroglu M, Ahmed R, Manev H (2003). "The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice". Neuropsychopharmacology. 28 (12): 2117–23. doi:10.1038/sj.npp.1300254. PMID 12865893.
- ^ Uz T, Dimitrijevic N, Akhisaroglu M, Imbesi M, Kurtuncu M, Manev H (2004). "The pineal gland and anxiogenic-like action of fluoxetine in mice". NeuroReport. 15 (4): 691–4. doi:10.1097/00001756-200403220-00023. PMID 15094477.
- ^ Manev H, Uz T, Kharlamov A, Joo J (1996). "Increased brain damage after stroke or excitotoxic seizures in melatonin-deficient rats". FASEB J. 10 (13): 1546–51. PMID 8940301.
- ^ Sharan K, Lewis K, Furukawa T, Yadav VK (2017). "Regulation of bone mass through pineal-derived melatonin-MT2 pathway". J Pineal Res. 79A (2): 263–270. doi:10.1002/jbm.a.30786. PMID 28512916.
- ^ a b c Zimmerman, Robert A. "Age-Related Incidence of Pineal Calcification Detected by Computed Tomography" (PDF). Radiological Society of North America. Archived from the original (PDF) on 24 March 2012. Retrieved 21 June 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Luke, Jennifer (March–April 2001). "Fluoride Deposition in the Aged Human Pineal Gland". Caries Res. 2001 (35): 125–128. doi:10.1159/000047443. PMID 11275672.
{{cite journal}}: Cite has empty unknown parameters:|laysummary=,|laydate=, and|laysource=(help) - ^ "The Pineal Body". Human Anatomy (Gray's Anatomy). Archived from the original on 26 August 2011. Retrieved 7 September 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Mahlberg, R.; Walther, S.; Kalus, P.; Bohner, G.; Haedel, S.; Reischies, F. M.; Kühl, K. P.; Hellweg, R.; Kunz, D. (2008). "Pineal calcification in Alzheimer's disease: An in vivo study using computed tomography". Neurobiology of Aging. 29 (2): 203–209. doi:10.1016/j.neurobiolaging.2006.10.003. PMID 17097768.
- ^ [rwevandsmd.com "RW Evans M.D."] Retrieved 26 December 2016.
{{cite web}}: Check|url=value (help) - ^ Robbins & Cotran Pathologic Basis of Disease. p. 1137. ISBN 9780323296359.
- ^ Bruce, Jeffrey. "Pineal Tumours". eMedicine. Archived from the original on 5 September 2015. Retrieved 25 September 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Pineal Tumours". American Brain Tumour Association. Archived from the original on 26 September 2015. Retrieved 25 September 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Clark, Aaron J.; Sughrue, Michael E.; Ivan, Michael E.; Aranda, Derick; Rutkowski, Martin J.; Kane, Ari J.; Chang, Susan; Parsa, Andrew T. (2010). "Factors influencing overall survival rates for patients with pineocytoma". Journal of Neuro-Oncology. 100 (2): 255–260. doi:10.1007/s11060-010-0189-6. PMC 2995321. PMID 20461445.
- ^ Cole, W.; Youson, J. (1982). "Morphology of the pineal complex of the anadromous sea lamprey, Petromyzon marinus L". Developmental Dynamics. 165 (2): 131–163. doi:10.1002/aja.1001650205.
- ^ Cope, Edward Drinker (1888). "The pineal eye in extinct vertebrates". American Naturalist. 22 (262): 914–917. doi:10.1086/274797.
- ^ Schultze, Hans-Peter (1993). "Patterns of diversity in the skulls of jawed fishes". In Hanken, James; Hall, Brian K. (eds.). The Skull, Volume 2: Patterns of Structural and Systematic Diversity. Chicago, Illinois: University of Chicago Press. pp. 189–254. ISBN 9780226315683. Retrieved 2 February 2017.
- ^ Dodt, Eberhard (1973). "The parietal eye (pineal and parietal organs) of lower vertebrates". Visual Centers in the Brain. Springer. pp. 113–140.
- ^ Tosini, Gianluca (1997). "The pineal complex of reptiles: physiological and behavioral roles". Ethology Ecology & Evolution. 9 (4): 313–333. doi:10.1080/08927014.1997.9522875.
- ^ Dendy, A. (1911). "On the structure, development and morphological interpretation of the pineal organs and adjacent parts of the brain in the tuatara (Sphenodon punctatus)". Philosophical Transactions of the Royal Society of London B. 201 (274–281): 227–331. doi:10.1098/rstb.1911.0006.
- ^ a b Quay, WB (1979). "The parietal eye–pineal complex". In Gans, Carl; Northcutt, R. Glenn; Ulinski, Philip (eds.). Biology of the Reptilia. Volume 9. Neurology A. London: Academic Press. pp. 245–406. Archived from the original on 3 February 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Vollrath, L. (1979). "Comparative morphology of the vertebrate pineal complex". Progress in Brain Research. Progress in Brain Research. 52: 25–38. doi:10.1016/S0079-6123(08)62909-X. ISBN 9780444801142. PMID 398532.
- ^ a b Ralph, Charles (1975). "The pineal gland and geographical distribution of animals". International Journal of Biometeorology. 19 (4): 289–303. doi:10.1007/bf01451040. PMID 1232070.
- ^ Ralph, Charles; Young, S; Gettinger, R; O'Shea, TJ (1985). "Does the manatee have a pineal body?". Acta Zoologica. 66: 55–60. doi:10.1111/j.1463-6395.1985.tb00647.x.
- ^ Adler, Kraig (1976). "Extraocular photoreception in amphibians". Photochemistry and Photobiology. 23 (4): 275–298. doi:10.1111/j.1751-1097.1976.tb07250.x.
- ^ Natesan A, Geetha L, Zatz M (2002). "Rhythm and soul in the avian pineal" (PDF). Cell Tissue Res. 309 (1): 35–45. doi:10.1007/s00441-002-0571-6. PMID 12111535.
- ^ Moore RY, Heller A, Wurtman RJ, Axelrod J (January 1967). "Visual pathway mediating pineal response to environmental light". Science. 155 (759): 220–3. Bibcode:1967Sci...155..220M. doi:10.1126/science.155.3759.220. PMID 6015532.
- ^ Edinger, Tilly (1955). "The size of parietal foramen and organ in reptiles: a rectification". Bulletin of the Museum of Comparative Zoology. 114: 1–34. Archived from the original on 1 December 2017.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Kurochkin, Evgeny N.; Gareth J. Dyke; Sergei V. Saveliev; Evgeny M. Pervushov; Evgeny V. Popov (June 2007). "A fossil brain from the Cretaceous of European Russia and avian sensory evolution". Biology Letters. 3 (3). The Royal Society: 309–313. doi:10.1098/rsbl.2006.0617. PMC 2390680. PMID 17426009.
- ^ Descartes R. "The Passions of the Soul" excerpted from "Philosophy of the Mind," Chalmers, D. New York: Oxford University Press, Inc.; 2002. ISBN 978-0-19-514581-6
- ^ Wikisource:Ethics (Spinoza)/Part 5
- ^ Hollier, D, Against Architecture: The Writings of Georges Bataille, trans. Betsy Wing, MIT, 1989.
- ^ Bataille, G, Visions of Excess: Selected Writings, 1927–1939 (Theory and History of Literature, Vol 14), trans. Allan Stoekl et al., Manchester University Press, 1985
- ^ Strassman, Rick (2000). DMT: The Spirit Molecule. Inner Traditions. ISBN 1594779732. Archived from the original on 6 January 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Barker SA, Borjigin J, Lomnicka I, Strassman R (July 2013). "LC/MS/MS analysis of the endogenous dimethyltryptamine hallucinogens, their precursors, and major metabolites in rat pineal gland microdialysate". Biomed Chromatogr. 27 (12): 1690–1700. doi:10.1002/bmc.2981. PMID 23881860.
- ^ Lerner AB, Case JD, Takahashi Y (1960). "Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands". J Biol Chem. 235: 1992–7. PMID 14415935.
- ^ Coates, Paul M. (2005). Encyclopedia of Dietary Supplements. Marc R. Blackman, Gordon M. Cragg, Mark Levine, Joel Moss, Jeffrey D. White. CRC Press. p. 457. ISBN 0-8247-5504-9. Retrieved 31 March 2009.
External links
- gland Stained brain slice images which include the "pineal gland" at the BrainMaps project
- hier-280 at NeuroNames
- Histology at BU: Endocrine System: pineal gland (illustration)
- Anatomy Atlases, Microscopic atlas: Pineal gland
- MedPix: Images of Pineal region
- Studies of chinese original quiet sitting by using functional magnetic resonance imaging..
- Detection of nighttime melatonin level in Chinese Original Quiet Sitting.