Aldosterone: Difference between revisions
m Reverted edits by 124.179.77.125 (talk) to last version by Addbot |
No edit summary |
||
| Line 46: | Line 46: | ||
}} |
}} |
||
}} |
}} |
||
'''Aldosterone''' is a <!--yellow - under what circumstance is it yellow?--> [[steroid hormone]] ([[mineralocorticoid]] family) produced by the outer section ([[zona glomerulosa]]) of the [[adrenal cortex]] in the [[adrenal gland]]. It acts mainly on the [[distal tubule]]s and [[collecting duct]]s of the [[nephron]], the functional unit of the [[kidney]], to cause the conservation of [[sodium]], |
'''Aldosterone''' is a <!--yellow - under what circumstance is it yellow?--> [[steroid hormone]] ([[mineralocorticoid]] family) produced by the outer section ([[zona glomerulosa]]) of the [[adrenal cortex]] in the [[adrenal gland]]. It acts mainly on the [[distal tubule]]s and [[collecting duct]]s of the [[nephron]], the functional unit of the [[kidney]], to cause the conservation of [[sodium]], excretion of [[potassium]], increased water retention, and increased [[blood pressure]]. The overall effect of aldosterone is to increase reabsorption of ions and water in the kidney -- increasing blood volume and, therefore, increasing blood pressure. Aldosterone has exactly the opposite function of the [[atrial natriuretic hormone]] secreted by the [[heart]].<ref>Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14</ref> |
||
Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives. One example is [[spironolactone]], which lowers blood pressure by blocking the aldosterone receptor; its net effect is to reduce sodium and water retention, but increase retention of potassium. Aldosterone is part of the [[renin-angiotensin-aldosterone system|renin-angiotensin system]]. |
Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives. One example is [[spironolactone]], which lowers blood pressure by blocking the aldosterone receptor; its net effect is to reduce sodium and water retention, but increase retention of potassium. Aldosterone is part of the [[renin-angiotensin-aldosterone system|renin-angiotensin system]]. |
||
Revision as of 14:18, 26 March 2013
| |
| Names | |
|---|---|
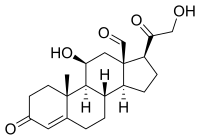
| IUPAC name
11β,21-Dihydroxy-3,20-dioxopregn-4-en-18-al
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.128 |
| KEGG | |
| MeSH | Aldosterone |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H28O5 | |
| Molar mass | 360.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Aldosterone is a steroid hormone (mineralocorticoid family) produced by the outer section (zona glomerulosa) of the adrenal cortex in the adrenal gland. It acts mainly on the distal tubules and collecting ducts of the nephron, the functional unit of the kidney, to cause the conservation of sodium, excretion of potassium, increased water retention, and increased blood pressure. The overall effect of aldosterone is to increase reabsorption of ions and water in the kidney -- increasing blood volume and, therefore, increasing blood pressure. Aldosterone has exactly the opposite function of the atrial natriuretic hormone secreted by the heart.[1]
Drugs that interfere with the secretion or action of aldosterone are in use as antihypertensives. One example is spironolactone, which lowers blood pressure by blocking the aldosterone receptor; its net effect is to reduce sodium and water retention, but increase retention of potassium. Aldosterone is part of the renin-angiotensin system.
Its activity is reduced in Addison's disease and increased in Conn's syndrome.
It was first isolated by Simpson and Tait in 1953.[2]
Synthesis
The corticosteroids are synthesized from cholesterol within the adrenal cortex. Most steroidogenic reactions are catalysed by enzymes of the cytochrome P450 family. They are located within the mitochondria and require adrenodoxin as a cofactor (except 21-hydroxylase and 17α-hydroxylase).
Aldosterone and corticosterone share the first part of their biosynthetic pathways. The last parts are mediated either by the aldosterone synthase (for aldosterone) or by the 11β-hydroxylase (for corticosterone). These enzymes are nearly identical (they share 11β-hydroxylation and 18-hydroxylation functions), but aldosterone synthase is also able to perform a 18-oxidation. Moreover, aldosterone synthase is found within the zona glomerulosa at the outer edge of the adrenal cortex; 11β-hydroxylase is found in the zona fasciculata and reticularis.
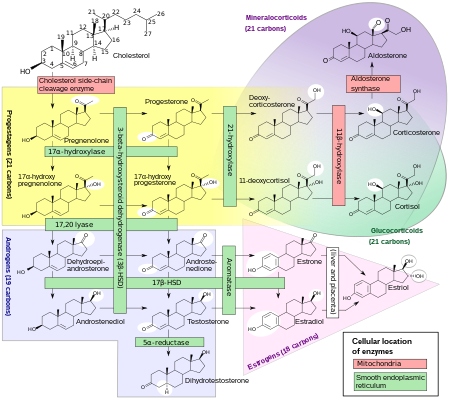
Note: aldosterone synthase is absent in other sections of the adrenal gland.
Stimulation
Aldosterone synthesis is stimulated by several factors:
- increase in the plasma concentration of angiotensin III, a metabolite of angiotensin II
- increase in plasma angiotensin II, ACTH, or potassium levels, which are present in proportion to plasma sodium deficiencies. (The increased potassium level works to regulate aldosterone synthesis by depolarizing the cells in the zona glomerulosa, which opens the voltage-dependent calcium channels.) The level of angiotensin II is regulated by angiotensin I, which is in turn regulated by the hormone renin. Potassium levels are the most sensitive stimulator of aldosterone.
- the ACTH stimulation test, which is sometimes used to stimulate the production of aldosterone along with cortisol to determine whether primary or secondary adrenal insufficiency is present. However, ACTH has only a minor role in regulating aldosterone production; with hypopituitarism there is no atrophy of the zona glomerulosa.
- plasma acidosis
- the stretch receptors located in the atria of the heart. If decreased blood pressure is detected, the adrenal gland is stimulated by these stretch receptors to release aldosterone, which increases sodium reabsorption from the urine, sweat, and the gut. This causes increased osmolarity in the extracellular fluid, which will eventually return blood pressure toward normal.
- adrenoglomerulotropin, a lipid factor, obtained from pineal extracts. It selectively stimulates secretion of aldosterone.[3]
The secretion of aldosterone has a diurnal rhythm.[4]
Function
Aldosterone is the primary of several endogenous members of the class of mineralocorticoids in humans. Deoxycorticosterone is another important member of this class. Aldosterone tends to promote Na+ and water retention, and lower plasma K+ concentration by the following mechanisms:
- Acting on the nuclear mineralocorticoid receptors (MR) within the principal cells of the distal tubule and the collecting duct of the kidney nephron, it upregulates and activates the basolateral Na+/K+ pumps, which pumps three sodium ions out of the cell and two potassium ions into the cell. This results in reabsorption of sodium (Na+) ions and water (which follows sodium) into the blood, and secreting potassium (K+) ions into the urine (lumen of collecting duct).
- Aldosterone upregulates epithelial sodium channels (ENaCs), increasing apical membrane permeability for Na+.
- Cl- is reabsorbed in conjunction with sodium cations to maintain the system's electrochemical balance.
- Aldosterone stimulates the secretion of K+ into the tubular lumen. [5]
- Aldosterone stimulates Na+ and water reabsorption from the gut, salivary and sweat glands in exchange for K+.
- Aldosterone stimulates secretion of H+ in exchange for Na+ in the intercalated cells of the cortical collecting tubules, regulating plasma bicarbonate (HCO3−) levels and its acid/base balance.[6]
Aldosterone is responsible for the reabsorption of about 2% of filtered sodium in the kidneys, which is nearly equal to the entire sodium content in human blood under normal glomerular filtration rates.[7]
Aldosterone, most probably acting through mineralocorticoid receptors, may positively influence neurogenesis in the dentate gyrus.[8]
Location of receptors
Steroid receptors are intracellular. The aldosterone mineralcorticoid receptor complex binds on the DNA to specific hormone response element, which leads to gene specific transcription.
Some of the transcribed genes are crucial for transepithelial sodium transport, including the three subunits of the epithelial sodium channel (ENaC), the Na+/K+ pumps and their regulatory proteins serum and glucocorticoid-induced kinase, and channel-inducing factor, respectively.
The mineralcorticoid receptor is stimulated by both aldosterone and cortisol, but a mechanism protects the body from excess aldosterone receptor stimulation by glucocorticoids (such as cortisol), which happen to be present at much higher concentrations than mineralcorticoids in the healthy individual. The mechanism consists of an enzyme called 11 β-hydroxysteroid dehydrogenase (11 β-HSD). This enzyme co-localizes with intracellular adrenal steroid receptors and converts cortisol into cortisone, a relatively inactive metabolite with little affinity for the MR. Liquorice, which contains glycyrrhetinic acid, can inhibit 11 β-HSD and lead to a mineralcorticoid excess syndrome.
Control of aldosterone release from the adrenal cortex

Major regulators
The role of the renin-angiotensin system
Angiotensin is involved in regulating aldosterone and is the core regulation.[10] Angiotensin II acts synergistically with potassium, and the potassium feedback is virtually inoperative when no angiotensin II is present.[11] A small portion of the regulation resulting from angiotensin II must take place indirectly from decreased blood flow through the liver due to constriction of capillaries.[12] When the blood flow decreases so does the destruction of aldosterone by liver enzymes.
The plasma concentration of potassium
The amount of aldosterone secreted is a direct function of the serum potassium [13][14] as probably determined by sensors in the carotid artery.[15][16]
ACTH
ACTH, a pituitary peptide, also has some stimulating effect on aldosterone, probably by stimulating the formation of deoxycorticosterone, a precursor of aldosterone.[17] Aldosterone is increased by blood loss,[18] pregnancy,[19] and possibly by other circumstances such as physical exertion, endotoxin shock, and burns.[20][21]
Miscellaneous regulators
The role of sympathetic nerves
The aldosterone production is also affected to one extent or another by nervous control, which integrates the inverse of carotid artery pressure,[15] pain, posture,[19] and probably emotion (anxiety, fear, and hostility) [22] (including surgical stress).[23] Anxiety increases aldosterone,[22] which must have evolved because of the time delay involved in migration of aldosterone into the cell nucleus.[24] Thus, there is an advantage to an animal's anticipating a future need from interaction with a predator, since too high a serum content of potassium has very adverse effects on nervous transmission.
The role of baroreceptors
Pressure sensitive baroreceptors are found in the vessel walls of nearly all large arteries in the thorax and neck, but are particularly plentiful in the sinuses of the carotid arteries and in the arch of the aorta. These specialized receptors are sensitive to changes in mean arterial pressure. An increase in sensed pressure results in an increased rate of firing by the baroreceptors and a negative feedback response, lowering systemic arterial pressure. Aldosterone release causes sodium and water retention, which causes increased blood volume, and a subsequent increase in blood pressure, which is sensed by the baroreceptors.[25] To maintain normal homeostasis these receptors also detect low blood pressure or low blood volume, causing aldosterone to be released. This results in sodium retention in the kidney, leading to water retention and increased blood volume.[26]
The plasma concentration of sodium
Aldosterone is a function of the inverse of the sodium intake as sensed via osmotic pressure.[27] The slope of the response of aldosterone to serum potassium is almost independent of sodium intake.[28] Aldosterone is much increased at low sodium intakes, but the rate of increase of plasma aldosterone as potassium rises in the serum is not much lower at high sodium intakes than it is at low. Thus, the potassium is strongly regulated at all sodium intakes by aldosterone when the supply of potassium is adequate, which it usually is in primitive diets.
Aldosterone feedback
Feedback by aldosterone concentration itself is of a nonmorphological character (that is, other than changes in the cells' number or structure) and is poor, so the electrolyte feedbacks predominate, short term.[20]
Associated clinical conditions
Hyperaldosteronism is abnormally increased levels of aldosterone, while hypoaldosteronism is abnormally decreased levels of aldosterone.
| Upper limits of plasma aldosterone reference ranges | ||
|---|---|---|
| Upright adult, 8-10 am | 34.0[29] | ng/dL |
| 940[30] | pmol/L | |
| Supine adult, 8-10 am | 19.0[29] | ng/dL |
| 530[30] | pmol/L | |
| Upright adult, 4-6 pm | 23.0[29] | ng/dL |
| 640[30] | pmol/L | |
A measurement of aldosterone in blood may be termed a plasma aldosterone concentration (PAC), which may be compared to plasma renin activity (PRA) as an aldosterone-to-renin ratio.
Hyperaldosteronism
Primary aldosteronism, also known as primary hyperaldosteronism, is characterized by the overproduction of aldosterone by the adrenal glands,[31] when not a result of excessive renin secretion. It leads to arterial hypertension (high blood pressure) associated with hypokalemia, usually a diagnostic clue. Secondary hyperaldosteronism, on the other hand, is due to overactivity of the renin-angiotensin system.
Conn's syndrome is primary hyperaldosteronism caused by an aldosterone-producing adenoma.
Depending on cause and other factors, hyperaldosteronism can be treated by surgery and/or medically, such as by aldosterone antagonists.
Hypoaldosteronism
An ACTH stimulation test for aldosterone can help in determining the cause of hypoaldosteronism, with a low aldosterone response indicating a primary hypoaldosteronism of the adrenals, while a large response indicating a secondary hypoaldosteronism.
Additional images
-
Corticosteroid biosynthetic pathway in rat
See also
References
- ^ Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14
- ^ Williams JS, Williams GH (2003). "50th anniversary of aldosterone". J Clin Endocrinol Metab. 88 (6): 2364–72. doi:10.1210/jc.2003-030490. PMID 12788829.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Farrell G (1960). "Adrenoglomerulotropin". Circulation. 21 (5): 1009–15. doi:10.1161/01.CIR.21.5.1009. PMID 13821632.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hurwitz S, Cohen RJ, Williams GH (2004). "Diurnal variation of aldosterone and plasma renin activity: timing relation to melatonin and cortisol and consistency after prolonged [[bed rest]]". J App Physiol. 96 (4): 1406–14. doi:10.1152/japplphysiol.00611.2003. PMID 14660513.
{{cite journal}}: URL–wikilink conflict (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ unknown, unknown. "unknown". ionchannels.org. Retrieved 8/4/2011.
{{cite web}}: Check date values in:|accessdate=(help); Cite uses generic title (help) - ^ Rector, Floyd C.; Brenner, Barry M. (2004). Brenner & Rector's the kidney. Philadelphia: Saunders. ISBN 0-7216-0164-2. OCLC 51838812.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Sherwood, Lauralee (2001). Human physiology: from cells to systems. Pacific Grove, CA: Brooks/Cole. ISBN 0-534-56826-2. OCLC 43702042.
- ^ Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czéh B (2002). "The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat". Brain Res. 947 (2): 290–3. doi:10.1016/S0006-8993(02)03042-1. PMID 12176172.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Page 866-867 (Integration of Salt and Water Balance) and 1059 (The Adrenal Gland) in: Walter F., PhD. Boron (2003). Medical Physiology: A Cellular And Molecular Approaoch. Elsevier/Saunders. p. 1300. ISBN 1-4160-2328-3.
- ^ Williams GH, Dluhy RG (1972). "Aldosterone biosynthesis. Interrelationship of regulatory factors". Am J Med. 53 (5): 595–605. doi:10.1016/0002-9343(72)90156-8. PMID 4342886.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Pratt JH (1982). "Role of angiotensin II in potassium-mediated stimulation of aldosterone secretion in the dog". J Clin Invest. 70 (3): 667–72. doi:10.1172/JCI110661. PMC 370270. PMID 6286729.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Messerli FH, Nowaczynski W, Honda M; et al. (1977). "Effects of angiotensin II on steroid metabolism and hepatic blood flow in man". Circulation Research. 40 (2): 204–7. doi:10.1161/01.RES.40.2.204. PMID 844145.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bauer JH, Gauntner WC (1979). "Effect of potassium chloride on plasma renin activity and plasma aldosterone during sodium restriction in normal man". Kidney Int. 15 (3): 286–93. doi:10.1038/ki.1979.37. PMID 513492.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Linas SL, Peterson LN, Anderson RJ, Aisenbrey GA, Simon FR, Berl T (1979). "Mechanism of renal potassium conservation in the rat". Kidney Int. 15 (6): 601–11. doi:10.1038/ki.1979.79. PMID 222934.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Gann DS Mills IH Bartter 1960 On the hemodynamic parameter mediating increase in aldosterone secretion in the dog. Fed. Proceedings 19; 605-610.
- ^ Gann DS, Cruz JF, Casper AG, Bartter FC (1962). "Mechanism by which potassium increases aldosterone secretion in the dog". Am J Physiol. 202: 991–6. PMID 13896654.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brown RD, Strott CA, Liddle GW (1972). "Site of stimulation of aldosterone biosynthesis by angiotensin and potassium". J Clin Invest. 51 (6): 1413–8. doi:10.1172/JCI106937. PMC 292278. PMID 4336939.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ruch TC Fulton JF 1960 Medical Physiology and Biophysics. W.B. Saunders and Co., Phijl & London. On p1099.
- ^ a b Farrell G (1958). "Regulation of aldosterone secretion". Physiological Reviews. 38 (4): 709–28. PMID 13590935.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Vecsei, Pál; Gláz, Edith (1971). Aldosterone. New York: Pergamon Press. ISBN 0-08-013368-1. OCLC 186705.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Farrell GL, Rauschkolb EW (1956). "Evidence for diencephalic regulation of aldosterone secretion". Endocrinology. 59 (5): 526–31. doi:10.1210/endo-59-5-526. PMID 13375573.
{{cite journal}}: Unknown parameter|month=ignored (help) on 529 - ^ a b Venning EH, DyrenfurthY I, Beck JC (1957). "Effect of anxiety upon aldosterone excretion in man". J Clin Endocrinol Metab. 17 (8): 1005–8. doi:10.1210/jcem-17-8-1005. PMID 13449153.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Elman R, Shatz BA, Keating RE, Weichselbaum TE (1952). "Intracellular and Extracellular Potassium Deficits in Surgical Patients". Annals of surgery. 136 (1): 111–31. doi:10.1097/00000658-195208000-00013. PMC 1802239. PMID 14934025.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sharp GUG Leaf A 1966 in; Recent Progress in Hormone Research.(Pincus G, ed.
- ^ Copstead, E. C. & Banasik, J. L. (2010.) Pathophysiology. (4th ed.). St. Louis, Mo: Saunders Elsevier.
- ^ Marieb, E. N. (2004) Human anatomy and physiology (6th ed) San Francisco: Pearson Benjamin Cummings.
- ^ Schneider EG, Radke KJ, Ulderich DA, Taylor RE (1985). "Effect of osmolality on aldosterone secretion". Endocrinology. 116 (4): 1621–6. doi:10.1210/endo-116-4-1621. PMID 3971930.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dluhy RG, Axelrod L, Underwood RH, Williams GH (1972). "Studies of the control of plasma aldosterone concentration in normal man: II. Effect of dietary potassium and acute potassium infusion". J Clin Invest. 51 (8): 1950–7. doi:10.1172/JCI107001. PMC 292351. PMID 5054456.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c New Assays for Aldosterone, Renin and Parathyroid Hormone University of Washington, Department of Laboratory Medicine. Retrieved Mars 2011
- ^ a b c Converted from mass values using molar mass of 360.44 g/mol
- ^ Conn JW, Louis LH (1955). "Primary aldosteronism: a new clinical entity". Trans. Assoc. Am. Physicians. 68: 215–31, discussion, 231–3. PMID 13299331.