Glyphosate: Difference between revisions
WHO: carcinogenic |
Undid revision 653525706 by DePiep (talk) Seems fine to keep in the body for now per WP:RECENTISM with discussion in sources still ongoing about the WHO's statement. Best to give it some time. |
||
| Line 66: | Line 66: | ||
}} |
}} |
||
}} |
}} |
||
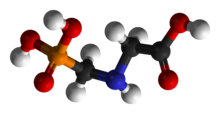
'''Glyphosate''' (''N''-(phosphonomethyl)glycine) is a broad-spectrum [[Herbicide#Classification|systemic herbicide]] used to kill [[weed]]s, especially annual broadleaf weeds and grasses known to compete with commercial crops grown around the globe. It was discovered to be a herbicide by [[Monsanto]] chemist [[John E. Franz]] in 1970.<ref>{{ cite patent | country = US | number = 3799758 | status =patent | title = N-phosphonomethyl-glycine phytotoxicant compositions | gdate = 1974-03-26 | fdate = 1971-08-09 | inventor = Franz JE | assign1 = Monsanto Company }}</ref> Monsanto brought it to market in the 1970s under the trade name '''Roundup''', and Monsanto's last commercially relevant United States [[patent]] expired in 2000. |
'''Glyphosate''' (''N''-(phosphonomethyl)glycine) is a broad-spectrum [[Herbicide#Classification|systemic herbicide]] used to kill [[weed]]s, especially annual broadleaf weeds and grasses known to compete with commercial crops grown around the globe. It was discovered to be a herbicide by [[Monsanto]] chemist [[John E. Franz]] in 1970.<ref>{{ cite patent | country = US | number = 3799758 | status =patent | title = N-phosphonomethyl-glycine phytotoxicant compositions | gdate = 1974-03-26 | fdate = 1971-08-09 | inventor = Franz JE | assign1 = Monsanto Company }}</ref> Monsanto brought it to market in the 1970s under the trade name '''Roundup''', and Monsanto's last commercially relevant United States [[patent]] expired in 2000. |
||
Glyphosate was quickly adopted by farmers, even more so when Monsanto introduced glyphosate-resistant [[genetically modified crops|crops]], enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States agricultural sector, with 180 to 185 million pounds ({{convert|180000000|to|185000000|lb|t|abbr=off|disp=output only}}) applied, and the second-most used in home and garden market where users applied 5 to 8 million pounds ({{convert|5000000|to|8000000|lb|t|abbr=off|disp=output only}}); in addition, industry, commerce, and government applied 13 to 15 million pounds ({{convert|13000000|to|15000000|lb|t|abbr=off|disp=output only}}).<ref name="EPAusage">United States EPA 2007 Pesticide Market Estimates [http://www.epa.gov/opp00001/pestsales/07pestsales/usage2007_2.htm#3_6 Agriculture], [http://www.epa.gov/opp00001/pestsales/07pestsales/usage2007_3.htm#3_7 Home and Garden]</ref> With its heavy use in agriculture, weed resistance to glyphosate is a growing problem. While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide and are widely used, concerns about their effects on humans and the environment persist. |
Glyphosate was quickly adopted by farmers, even more so when Monsanto introduced glyphosate-resistant [[genetically modified crops|crops]], enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States agricultural sector, with 180 to 185 million pounds ({{convert|180000000|to|185000000|lb|t|abbr=off|disp=output only}}) applied, and the second-most used in home and garden market where users applied 5 to 8 million pounds ({{convert|5000000|to|8000000|lb|t|abbr=off|disp=output only}}); in addition, industry, commerce, and government applied 13 to 15 million pounds ({{convert|13000000|to|15000000|lb|t|abbr=off|disp=output only}}).<ref name="EPAusage">United States EPA 2007 Pesticide Market Estimates [http://www.epa.gov/opp00001/pestsales/07pestsales/usage2007_2.htm#3_6 Agriculture], [http://www.epa.gov/opp00001/pestsales/07pestsales/usage2007_3.htm#3_7 Home and Garden]</ref> With its heavy use in agriculture, weed resistance to glyphosate is a growing problem. While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide and are widely used, concerns about their effects on humans and the environment persist. |
||
Revision as of 23:12, 25 March 2015

| |
| |

| |
| Names | |
|---|---|
| IUPAC name
N-(phosphonomethyl)glycine
| |
| Other names
2-[(phosphonomethyl)amino]acetic acid
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.012.726 |
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H8NO5P | |
| Molar mass | 169.073 g·mol−1 |
| Appearance | white crystalline powder |
| Density | 1.704 (20 °C) |
| Melting point | 184.5 °C (364.1 °F; 457.6 K) |
| Boiling point | 187 °C (369 °F; 460 K) |
| 1.01 g/100 mL (20 °C) | |
| log P | −2.8 |
| Acidity (pKa) | <2, 2.6, 5.6, 10.6 |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H318, H411 | |
| P273, P280, P305+P351+P338, P310, P501 | |
| Flash point | Non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glyphosate (N-(phosphonomethyl)glycine) is a broad-spectrum systemic herbicide used to kill weeds, especially annual broadleaf weeds and grasses known to compete with commercial crops grown around the globe. It was discovered to be a herbicide by Monsanto chemist John E. Franz in 1970.[3] Monsanto brought it to market in the 1970s under the trade name Roundup, and Monsanto's last commercially relevant United States patent expired in 2000.
Glyphosate was quickly adopted by farmers, even more so when Monsanto introduced glyphosate-resistant crops, enabling farmers to kill weeds without killing their crops. In 2007, glyphosate was the most used herbicide in the United States agricultural sector, with 180 to 185 million pounds (82,000 to 84,000 tonnes) applied, and the second-most used in home and garden market where users applied 5 to 8 million pounds (2,300 to 3,600 tonnes); in addition, industry, commerce, and government applied 13 to 15 million pounds (5,900 to 6,800 tonnes).[4] With its heavy use in agriculture, weed resistance to glyphosate is a growing problem. While glyphosate and formulations such as Roundup have been approved by regulatory bodies worldwide and are widely used, concerns about their effects on humans and the environment persist.
Glyphosate's mode of action is to inhibit an enzyme involved in the synthesis of the aromatic amino acids: tyrosine, tryptophan, and phenylalanine. It is absorbed through foliage and translocated to growing points. Because of this mode of action, it is only effective on actively growing plants; it is not effective as a pre-emergence herbicide.
Some crops have been genetically engineered to be resistant to glyphosate (i.e., Roundup Ready, also created by Monsanto Company). Such crops allow farmers to use glyphosate as a postemergence herbicide against both broadleaf and cereal weeds, but the development of similar resistance in some weed species is emerging as a costly problem. Roundup Ready soybean was the first Roundup Ready crop.
Discovery
Glyphosate was first synthesized in 1950 by Swiss chemist Henry Martin, who worked for the Swiss company Cilag. The work was never published.[5]: 1 In a case of parallel invention, glyphosate was independently discovered at Monsanto in 1970. Monsanto chemists had synthesized about 100 analogs of aminomethylphosphonic acid as potential water-softening agents. Two were found to have weak herbicidal activity, and John E. Franz, a chemist at Monsanto, was asked to try to make analogs with stronger herbicidal activity. Glyphosate was the third analog he made.[5]: 1–2 [6]
Glyphosate has been called by experts in herbicides "virtually ideal" due to its broad spectrum and low toxicity to animal life compared with other herbicides.[7][8][9][10] Franz received the National Medal of Technology in 1987[11] and the Perkin Medal for Applied Chemistry[12] in 1990 for his discoveries. Franz was inducted into the National Inventor's Hall of Fame in 2007.[13]
Chemistry

Glyphosate is an aminophosphonic analogue of the natural amino acid glycine, and the name is a contraction of gly(cine) phos(phon)ate. The molecule has several dissociable hydrogens, especially the first hydrogen of the phosphate group. The molecule tends to exist as a zwitterion where a phosphonic hydrogen dissociates and joins the amine group. Glyphosate is soluble in water to 12 g/l at room temperature.

The main deactivation path is hydrolysis to aminomethylphosphonic acid (AMPA).[14]
Biochemistry
Glyphosate kills plants by interfering with the synthesis of the aromatic amino acids phenylalanine, tyrosine, and tryptophan. It does this by inhibiting the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS), which catalyzes the reaction of shikimate-3-phosphate (S3P) and phosphoenolpyruvate to form 5-enolpyruvyl-shikimate-3-phosphate (ESP).[15]
ESP is subsequently dephosphorylated to chorismate, an essential precursor for the amino acids mentioned above.[16] These amino acids are used in protein synthesis and to produce secondary metabolites such as folates, ubiquinones, and naphthoquinone.
X-ray crystallographic studies of glyphosate and EPSPS show that glyphosate functions by occupying the binding site of the phosphoenolpyruvate, mimicking an intermediate state of the ternary enzyme substrates complex.[17][18]
The commercially important enzyme that glyphosate inhibits, EPSPS, is found only in plants and micro-organisms. EPSPS is not present in animals, which instead obtain aromatic amino acids from their diets.[19]
Glyphosate is absorbed through foliage. Because of this mode of action, it is only effective on actively growing plants; it is not effective in preventing seeds from germinating.
Environmental fate
Glyphosate adsorbs strongly to soil and is not expected to move vertically below the six-inch soil layer; residues are expected to be immobile in soil. Glyphosate is readily degraded by soil microbes to aminomethylphosphonic acid (AMPA) and carbon dioxide. Glyphosate and AMPA are not likely to move to ground water due to their strong adsorptive characteristics. However, glyphosate does have the potential to contaminate surface waters due to its aquatic use patterns and through erosion, as it adsorbs to soil particles suspended in runoff. If glyphosate reaches surface water, it would not be broken down readily by water or sunlight.[20]
The half-life of glyphosate in soil ranges between 2 and 197 days; a typical field half-life of 47 days has been suggested. Soil and climate conditions affect glyphosate's persistence in soil. The median half-life of glyphosate in water varies from a few to 91 days.[21]
According to the National Pesticide Information Center fact sheet, glyphosate is not included in compounds tested for by the Food and Drug Administration's Pesticide Residue Monitoring Program, nor in the United States Department of Agriculture's Pesticide Data Program. However, a field test showed that lettuce, carrots, and barley contained glyphosate residues up to one year after the soil was treated with 3.71 lb of glyphosate per acre (4.15 kg per hectare).[21]
Use

Glyphosate is effective in killing a wide variety of plants, including grasses and broadleaf and woody plants. It has a relatively small effect on some clover species.[22] By volume, it is one of the most widely used herbicides.[21] It is commonly used for agriculture, horticulture, viticulture, and silviculture purposes, as well as garden maintenance (including home use).[21][23]
In many cities, glyphosate is sprayed along the sidewalks and streets, as well as crevices in between pavement where weeds often grow. However, up to 24% of glyphosate applied to hard surfaces can be run off by water.[24] Glyphosate contamination of surface water is attributed to urban and agricultural use.[25] Glyphosate is used to clear railroad tracks and get rid of unwanted aquatic vegetation.[26]
In addition to its use as an herbicide, glyphosate is also used for crop desiccation (siccation) to increase harvest yield[26] and, as a result of desiccation, to increase sucrose concentration in sugarcane before harvest.[27]
Formulations and tradenames
Glyphosate is marketed in the United States and worldwide by many agrochemical companies, in different solution strengths and with various adjuvants, under dozens of tradenames.[28][29][30][31] As of 2013, it was the world's largest-selling herbicide, and Chinese manufacturers collectively are the world's largest producers of glyphosate and its precursors.[32]
Manufacturers include Bayer, Dow AgroSciences, Du Pont, Cenex/Land O’Lakes, Helena, Monsanto, Platte, Riverside/Terra, and Zeneca.[31]
Glyphosate is an acid molecule, so it is formulated as a salt for packaging and handling. Various salt formulations include isopropylamine, diammonium, monoammonium, or potassium as the counterion. Some brands include more than one salt. Some companies report their product as acid equivalent (ae) of glyphosate acid, or some report it as active ingredient (ai) of glyphosate plus the salt, and others report both. To compare performance of different formulations, knowledge of how the products were formulated is needed. Since the salt does not contribute to weed control and different salts have different weights, the acid equivalent is a more accurate method of expressing and comparing concentrations.[33] Adjuvant loading refers to the amount of adjuvant[34][35] already added to the glyphosate product. Fully loaded products contain all the necessary adjuvants, including surfactant; some contain no adjuvant system, while other products contain only a limited amount of adjuvant (minimal or partial loading) and additional surfactants must be added to the spray tank before application.[33] As of 2000 (just before Monsanto's patent on glyphosate expired), over 400 commercial adjuvants from over 34 different companies were available for use in commercial agriculture.[36][37]
Products are supplied most commonly in formulations of 120, 240, 360, 480, and 680 g/l of active ingredient. The most common formulation in agriculture is 360 g/l, either alone or with added cationic surfactants.
For 360 g/l formulations, European regulations allow applications of up to 12 l/ha for control of perennial weeds such as couch grass. More commonly, rates of 3 l/ha are practiced for control of annual weeds between crops.[38]
Monsanto

Monsanto developed and patented the use of glyphosate to kill weeds in the 1970s, and has marketed it as Roundup since 1973. It retained exclusive rights in the United States until its patent expired in September, 2000.
As of 2009, sales of these herbicide products represented about 10% of Monsanto's revenue due to competition from other producers of other glyphosate-based herbicides;[39] their Roundup products (which include GM seeds) represented about half of Monsanto's gross margin.[40]
The active ingredient of the Monsanto herbicides is the isopropylamine salt of glyphosate. Another important ingredient in some formulations is the surfactant polyethoxylated tallow amine.
Monsanto also produces seeds which grow into plants genetically engineered to be tolerant to glyphosate. The genes contained in these seeds are patented. Such crops allow farmers to use glyphosate as a postemergence herbicide against most broadleaf and cereal weeds. Soy was the first glyphosate-resistant crop.
Toxicity
Glyphosate is the active ingredient in herbicide formulations containing it. However, in addition to glyphosate salts, commercial formulations of glyphosate contain additives such as surfactants which vary in nature and concentration. Laboratory toxicology studies have suggested that other ingredients in combination with glyphosate may have greater toxicity than glyphosate alone.[41] Toxicologists have studied glyphosate alone, additives alone, and formulations.
Glyphosate toxicity
Glyphosate has a United States Environmental Protection Agency (EPA) Toxicity Class of III (on a I to IV scale, where IV is least dangerous) for oral and inhalation exposure.[20] Thus, as with other herbicides, the EPA requires that products containing glyphosate carry a label that warns against oral intake, mandates the use of protective clothing, and instructs users not to re-enter treated fields for at least four hours.[20][42] Glyphosate does not bioaccumulate in mammals; it is excreted in urine and feces.[20] It breaks down variably quickly depending on the particular environment.
Human
Human acute toxicity is dose-related. Acute fatal toxicity has been reported in deliberate overdose.[41][43] Early epidemiological studies have not found associations between long-term low-level exposure to glyphosate and any disease.[44][45][46] Neither glyphosate nor typical glyphosphate-based formulations (GBFs) pose a genotoxicity risk in humans under normal conditions of human or environmental exposures.[47]
The EPA considers glyphosate to be noncarcinogenic and relatively low in dermal and oral acute toxicity.[20] The EPA considered a "worst case" dietary risk model of an individual eating a lifetime of food derived entirely from glyphosate-sprayed fields with residues at their maximum levels. This model indicated that no adverse health effects would be expected under such conditions.[20]
The European Commission's review of the data conducted in 2002 concluded equivocal evidence existed of a relationship between glyphosate exposure during pregnancy and cardiovascular malformations; however, a review published in 2013 found the evidence "fails to support a potential risk for increased cardiovascular defects as a result of glyphosate exposure during pregnancy."[48]
A 2012 meta-analysis of all epidemiological studies of glyphosate exposure found no correlation with any kind of cancer.[49] A 2014 meta-analysis limited to epidemiological studies of workers who use pesticides found a correlation between occupational exposure to glyphosate and increased risk of B cell lymphoma, the most common kind of non-Hodgkin lymphoma (NHL). Workers exposed to glyphosate were about twice as likely to get B cell lymphoma.[50] Overall, around 2% of adults (including workers) are diagnosed with NHL at some point during their lifetime.[51]
In 2015, the International Agency for Research on Cancer named glyphosate as a probable carcinogen. [52] Monsanto's spokesman disagreed saying, "All labeled uses of glyphosate are safe for human health."[53]
Fish and amphibians
Glyphosate is generally less persistent in water than in soil, with 12- to 60-day persistence observed in Canadian pond water, yet because glyphosate binds to soil, persistence of over a year has been observed in the sediments of ponds in Michigan and Oregon.[20] In streams, maximum glyphosate concentrations were measured immediately after treatment and dissipated rapidly.[20] According to research done in the late 1980s and early 1990 (Ecotoxicological Risk Assessment for Roundup Herbicide), glyphosate in ecological exposures studied is "practically nontoxic to slightly toxic" for amphibians and fish.[54]
In a 2013 review, mixed results were observed in nonmammalian systems. Glyphosate and GBFs tend to elicit DNA damage effects at high or toxic dose levels, but the data suggest this is due to cytotoxicity rather than genotoxicity. GBF activity is perhaps associated with the associated surfactants.[47]
Soil, microorganisms and worms
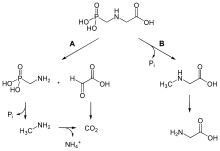
When glyphosate comes into contact with the soil, it can be rapidly bound to soil particles and be inactivated.[20][55] Unbound glyphosate can be degraded by bacteria.[56] Glyphosate and its degradation product, AMPA, residues are considered to be much more toxicologically and environmentally benign than most of the herbicides replaced by glyphosate.[57]
In soils, half-lives vary from as little as three days at a site in Texas to 141 days at a site in Iowa.[55] In addition, the glyphosate metabolite AMPA has been found in Swedish forest soils up to two years after a glyphosate application. In this case, the persistence of AMPA was attributed to the soil being frozen for most of the year.[58] Glyphosate adsorption to soil, and later release from soil, varies depending on the kind of soil.[59][60] A 2009 study using a RoundUp formulation concluded absorption into plants delays subsequent soil degradation and can increase glyphosate persistence in soil from two to six times.[61]
Laboratory studies published in 1991 and 1992 indicated GBFs could harm beneficial insects[62] and earthworms.[63] However, the reported effect of glyphosate on earthworms has been criticized.[54] The results conflict with results from field studies where no effects were noted for the number of nematodes, mites, or springtails after treatment with Roundup at 2 kg/ha of active ingredient.[64]
Glyphosate can harm the bacterial ecology of soil and cause micronutrient deficiencies in plants.[65] Other studies found that while "recommended dosages of glyphosate did not affect growth rates", much higher dosages reduced respiration in nitrogen-fixing bacteria.[66][67] A 2012 study on the effect of Roundup (glyphosate with adjuvants) on three microorganisms used in dairy products found, while the formulation had "a microbicide effect at lower concentrations than those recommended in agriculture", glyphosate alone "at these levels has no significant effect".[68]
Additive toxicity
This section needs expansion. You can help by adding to it. (September 2012) |
Glyphosate formulations may contain a number of adjuvants, the identity of which is considered a trade secret and not disclosed by government regulators. In the United States, the Federal Insecticide, Fungicide, and Rodenticide Act requires that all pesticides (including herbicides) be evaluated by the EPA prior to sale, including product’s chemistry, environmental fate, residue chemistry, dietary and nondietary hazards to humans, and hazards to domestic animals and nontarget organisms[69] These evaluations are performed for each active ingredient, each inert ingredient, and for the final product formulation. Additional evaluations are performed by the FDA to set permitted residue levels in food for pesticide products used on food crops.[70]
Surfactants
Surfactants lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. They may act as detergents, wetting agents, emulsifiers, foaming agents, and dispersants. As agricultural spray adjuvants, they may be included in a formulation sold to the consumer (in can), or may be bought separately and mixed on site (tank mix).
Polyethoxylated tallow amine (POEA) is part of the original Roundup formulation. Registration data in New Zealand showed Roundup contained 18% POEA. POEA is a surfactant that enhances the activity of herbicides such as glyphosate. The role of a surfactant in a herbicide product is to improve wetting of the surface of plants for maximum coverage and aid penetration through the plant surface. A review of the literature provided to the EPA in 1997 found POEA to be more toxic to fish than glyphosate.[71]
Spreader 90 is a surfactant used in tank mixes.[75] Spreader 90 contains 1,2 propanediol (also known as propylene glycol), propane 1,2,3 triol (also known as glycerol), alcohol ethoxylate, and dimethylpolysiloxane.[76][77] Of these ingredients, alcohol ethoxylates are among the widely used detergents in consumer products; commercial preparations are often mixes of homologs. Due to known toxicities to aquatic species, the Canadian recommended Federal Water Quality Guideline values are 70 µg/l.[78]
Formulation toxicity
Human
A 2000 review concluded that "under present and expected conditions of new use, there is no potential for Roundup herbicide to pose a health risk to humans".[79] A 2002 review by the European Union reached the same conclusion.[80]
Data from the California Environmental Protection Agency's Pesticide Illness Surveillance Program, which also tracks other agricultural chemicals, show glyphosate-related incidents are some of the most common.[81][82] However, incident counts alone do not take into account the number of people exposed and the severity of symptoms associated with each incident.[82] For example, if hospitalization were used as a measure of the severity of incidents, then glyphosate would be considered relatively safe; over a 13-year period in California, none of the 515 reported hospitalizations was attributed to glyphosate.[82]
Dermal exposure to ready-to-use glyphosate formulations can cause irritation, and photocontact dermatitis has been occasionally reported. These effects are probably due to the preservative benzisothiazolin-3-one. Severe skin burns are very rare.[41] Inhalation is a minor route of exposure, but spray mist may cause oral or nasal discomfort, an unpleasant taste in the mouth, or tingling and irritation in the throat. Eye exposure may lead to mild conjunctivitis. Superficial corneal injury is possible if irrigation is delayed or inadequate.[41]
Deliberate ingestion of Roundup in quantities ranging from 85 to 200 ml (of 41% solution) has resulted in death within hours of ingestion, although it has also been ingested in quantities as large as 500 ml with only mild or moderate symptoms.[83] A reasonable correlation is seen between the amount of Roundup ingested and the likelihood of serious systemic sequelae or death. Ingestion of more than 85 ml of the concentrated formulation is likely to cause significant toxicity in adults. Corrosive effects – mouth, throat and epigastric pain and dysphagia – are common. Renal and hepatic impairment are also frequent, and usually reflect reduced organ perfusion. Respiratory distress, impaired consciousness, pulmonary edema, infiltration on chest X-ray, shock, arrhythmias, renal failure requiring haemodialysis, metabolic acidosis, and hyperkalaemia may occur in severe cases. Bradycardia and ventricular arrhythmias often present prior to death.
Endocrine disruption
In a study of rats and mice fed diets of containing 0%, 0.3125%, 0.625%, 1.25%, 2.5%, or 5.0% glyphosate for 13 weeks, endocrine effects were observed only in rats and only at the two highest doses. Male rats at the highest dose exhibited reductions in sperm concentrations that remained within the strain's normal range. Female rats in the highest dose group experienced a slight increase in the length of the estrous cycle.[21]
Administering Roundup Transorb orally to prepubescent rats once a day for 30 days reduced testosterone production and affected testicle morphology, but did not affect levels of estradiol and corticosterone.[84]
In 2007, the EPA selected glyphosate for further screening through its Endocrine Disruptor Screening Program. Selection for this program is based on a compound's prevalence of use and does not imply particular suspicion of endocrine activity.[85]
Genetic damage
Several studies have not found mutagenic effects,[86] so glyphosate has not been listed in the U.S. EPA/IARC databases.[87] Various other studies suggest glyphosate may be mutagenic.[87]
Other animals
A review of the ecotoxicological data on Roundup shows at least 58 studies of the effects of Roundup itself on a range of organisms exist.[54] This review concluded, "for terrestrial uses of Roundup minimal acute and chronic risk was predicted for potentially exposed non-target organisms".
In reproductive toxicity studies performed in rats and rabbits, no adverse maternal or offspring effects were seen at doses below 175–293 mg/kg of body weight per day.[21]
The EPA,[20] the EC Health and Consumer Protection Directorate, and the UN World Health Organization have all concluded pure glyphosate is not carcinogenic. Opponents of glyphosate claim Roundup has been found to cause genetic damage, citing Peluso et al.[88] The authors concluded the damage was "not related to the active ingredient, but to another component of the herbicide mixture".
A 2003 study of various formulations of glyphosate found, "[the] risk assessments based on estimated and measured concentrations of glyphosate that would result from its use for the control of undesirable plants in wetlands and over-water situations showed that the risk to aquatic organisms is negligible or small at application rates less than 4 kg/ha and only slightly greater at application rates of 8 kg/ha.".[89] A 2013 meta-analysis also reviewed the available data related to potential impacts of glyphosate-based herbicides on amphibians. According to the authors, the use of glyphosate-based pesticides cannot be considered the major cause of amphibian decline, the bulk of which occurred prior to the widespread use of glyphosate or in pristine tropical areas with minimal glyphosate exposure. The authors recommended further study of species- and development-stage chronic toxicity, of environmental glyphosate levels, and ongoing analysis of data relevant to determining what if any role glyphosate might be playing in worldwide amphibian decline, and suggest including amphibians in standardized test batteries.[90]
Glyphosate formulations are much more toxic for amphibians and fish than glyphosate alone.[71] Glyphosate formulations may contain a number of so-called ‘inert’ ingredients or adjuvants, most of which are not publicly known as in many countries the law does not require that they be revealed.[91]
A study published in 2010 proposed commercial glyphosate can cause neural defects and craniofacial malformations in African clawed frogs (Xenopus laevis). The experiments used frog embryos that were incubated with 1:5000 dilutions of a commercial glyphosate solution. The frog embryos suffered diminution of body size, alterations of brain morphology, reduction of the eyes, alterations of the branchial arches and otic placodes, alterations of the neural plate, and other abnormalities of the nervous system. The authors suggested glyphosate itself was responsible for the observed results because injection of pure glyphosate produced similar results in a chicken model.[92]
Monsanto and other companies produce glyphosate products with alternative surfactants specifically formulated for aquatic use, for example the Monsanto products "Biactive" and "AquaMaster".[93][94] In 2001, the Monsanto product Vision® was studied in a forest wetlands site in Canada. Substantial mortality occurred only at concentrations exceeding the expected environmental concentrations as calculated by Canadian regulatory authorities. While it was found that site factors such as pH and suspended sediments substantially affected the toxicity in the amphibian larvae tested, overall, "results suggest that the silvicultural use of Vision herbicide in accordance with the product label and standard Canadian environmental regulations should have negligible adverse effects on sensitive larval life stages of native amphibians."[95]
Effect on plant health
A correlation was found between an increase in the infection rate of wheat by Fusarium head blight and the application of glyphosate, but "because of the nature of this study, we could not determine if the association between previous GF (glyphosate formulation) use and FHB development was a cause-effect relationship".[96] Other studies have found causal relationships between glyphosate and decreased disease resistance.[97]
Weed resistance
Resistance evolves after a weed population has been subjected to intense selection pressure in the form of repeated use of a single herbicide.[98][99] Weeds resistant to the herbicide have been called 'superweeds'.[100] The first documented cases of weed resistance to glyphosate were found in Australia in 1996, involving rigid ryegrass (Lolium rigidum) near Orange, New South Wales.[101][102] In 2006, farmers associations were reporting 103 biotypes of weeds within 63 weed species with herbicide resistance.[103] In 2009, Canada identified its first resistant weed, giant ragweed, and at that time 15 weed species had been confirmed as resistant to glyphosate.[98][104] As of 2010, in the United States 7 to 10 million acres (28,000 to 40,000 km2) of soil were afflicted by superweeds, or about 5% of the 170 million acres planted with corn, soybeans, and cotton, the crops most affected, in 22 states.[105] In 2012, Charles Benbrook reported that the Weed Science Society of America listed 22 super weeds in the U.S., with over 5.7 million has (14 million ac) infested by GR weeds and that Dow AgroSciences had carried out a survey and reported a figure of around 40 million ha (100 million ac).[106] As of 2014, the International Survey of Herbicide Resistant Weeds database listed 211 weeds that were resistant to glyphosate.[107]
In response to resistant weeds, farmers are hand-weeding, using tractors to turn over soil between crops, and using other herbicides in addition to glyphosate.
Palmer amaranth

In 2004, a glyphosate-resistant variation of Amaranthus palmeri, commonly known as Palmer amaranth, was found in Georgia and confirmed by a 2005 study.[108] In 2005, resistance was also found in North Carolina.[109] Widespread use of Roundup Ready crops led to an unprecedented selection pressure, and glyphosate resistance followed.[109] The weed variation is now widespread in the southeastern United States.[110] Cases have also been reported in Texas[110] and Virginia.[111]
Conyza

Conyza bonariensis (also known as hairy fleabane and buva) and Conyza canadensis (known as horseweed or marestail), are other weed species that had lately developed glyphosate resistance.[112][113][114] A 2008 study on the current situation of glyphosate resistance in South America concluded "resistance evolution followed intense glyphosate use" and the use of glyphosate-resistant soybean crops is a factor encouraging increases in glyphosate use.[115]
Ryegrass

Glyphosate-resistant ryegrass (Lolium) has occurred in most of the Australian agricultural areas and other areas of the world. All cases of evolution of resistance to glyphosate in Australia were characterized by intensive use of the herbicide while no other effective weed control practices were used. Studies indicate the resistant ryegrass does not compete well against nonresistant plants and their numbers decrease when not grown under conditions of glyphosate application.[116]
Johnson grass
Glyphosate-resistant Johnson grass (Sorghum halepense) is found in glyphosate-resistant soybean cultivation in northern Argentina.[117]
Legal cases
Advertising controversy
The New York Times reported that in 1996, "Dennis C. Vacco, the Attorney General of New York, ordered the company to pull ads that said Roundup was "safer than table salt" and "practically nontoxic" to mammals, birds and fish. The company withdrew the spots, but also said that the phrase in question was permissible under E.P.A. guidelines."[118]
Scientific fraud
On two occasions, the United States EPA has caught scientists deliberately falsifying test results at research laboratories hired by Monsanto to study glyphosate.[119] The first incident involved Industrial Biotest Laboratories (IBT). The United States Justice Department closed the laboratory in 1978, and its leadership was found guilty in 1983 of charges of falsifying statements, falsifying scientific data submitted to the government, and mail fraud.[120] In 1991, Don Craven, the owner of Craven Laboratories and three employees were indicted on 20 felony counts. Craven, along with fourteen employees were found guilty of similar crimes.[121]
Monsanto has stated the Craven Labs investigation was started by the EPA after a pesticide industry task force discovered irregularities, that the studies have been repeated, and that Roundup's EPA certification does not now use any studies from Craven Labs or IBT.[119]
Trade dumping allegations
United States companies have cited trade issues with glyphosate being dumped into the western world market areas by Chinese companies and a formal dispute was filed in 2010.[122][123]
Genetically modified crops
Some micro-organisms have a version of 5-enolpyruvoyl-shikimate-3-phosphate synthetase (EPSPS) resistant to glyphosate inhibition. A version of the enzyme that both was resistant to glyphosate and that was still efficient enough to drive adequate plant growth was identified by Monsanto scientists after much trial and error in an Agrobacterium strain called CP4, which was found surviving in a waste-fed column at a glyphosate production facility.[124][125][126]: 56 This CP4 EPSPS gene was cloned and transfected into soybeans. In 1996, genetically modified soybeans were made commercially available.[127] Current glyphosate-resistant crops include soy, maize (corn), canola, alfalfa, and cotton, with wheat still under development.
Genetically modified crops have become the norm in the United States. For example, in 2010, 70% of all the corn, 78% of cotton, and 93% of all soybeans planted were herbicide-resistant.[128]
See also
- 2,4-Dichlorophenoxyacetic acid
- Ammonium sulfamate
- Atrazine
- Environmental impact of pesticides
- Health effects of pesticides
- Integrated pest management
- Séralini affair
External links
- Pesticide Properties Database (PPDB) record for Glyphosate
- Pesticide Properties Database (PPDB) record for Glyphosate trimesium
- Pesticide Properties Database (PPDB) record for Glyphosate, isopropylamine salt
- Pesticide Properties Database (PPDB) record for Glyphosate, potassium salt
References
- ^ a b Glyphosate, Environmental Health Criteria monograph No. 159, Geneva: World Health Organization, 1994, ISBN 92-4-157159-4
- ^ Index no. 607-315-00-8 of Annex VI, Part 3, to Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJEU L353, 31.12.2008, pp 1–1355 at pp 570, 1100..
- ^ US patent 3799758, Franz JE, "N-phosphonomethyl-glycine phytotoxicant compositions", issued 1974-03-26, assigned to Monsanto Company
- ^ United States EPA 2007 Pesticide Market Estimates Agriculture, Home and Garden
- ^ a b Dill GM et al. Glyphosate: Discovery, Development, Applications, and Properties. Chapter 1 in Glyphosate Resistance in Crops and Weeds: History, Development, and Management, Vijay K. Nandula (Editor). Wiley, September 2010 ISBN 978-0-470-41031-8
- ^ Alibhai MF, Stallings WC (March 2001). "Closing down on glyphosate inhibition--with a new structure for drug discovery". Proc. Natl. Acad. Sci. U.S.A. 98 (6): 2944–6. Bibcode:2001PNAS...98.2944A. doi:10.1073/pnas.061025898. JSTOR 3055165. PMC 33334. PMID 11248008.
- ^ Stephen O Duke and Stephen B. Powles (2008) Glyphosate: a once-in-a-century herbicide: Mini-review. Pest Management Science Pest Manag Sci 64:319–325
- ^ Monsanto's John E. Franz Wins 1990 Perkin Medal Chem. Eng. News, 1990, 68 (11), pp 29–30 doi:10.1021/cen-v068n011.p029
- ^ Pesticide Action Network UK.Glyphosate fact sheet Pesticides News No.33, September 1996, p28-29 PAN-UK says it is "a welcome move away from chemicals which are highly toxic to humans and other non target organisms, and from chemicals which cause direct and lasting damage to the environment" and of course cautions against overuse.
- ^ Dr. Kathleen A. Marrs What is Biology Good For? Controlling Weeds: RoundUp
- ^ "The National Medal of Technology and Innovation Recipients - 1987". The United States Patent and Trademark Office. Retrieved 2012-011-29.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Stong C (May 1990). "People: Monsanto Scientist John E. Franz Wins 1990 Perkin Medal For Applied Chemistry". The Scientist. 4 (10): 28.
- ^ "Meet the 2007 National Inventors Hall of Fame Inductees". National Inventors Hall of Fame. 2007. Archived from the original on October 5, 2013.
- ^ Schuette J. "Environmental Fate of Glyphosate" (PDF). Department of Pesticide Regulation, State of California.
- ^ Steinrücken HC, Amrhein N (June 1980). "The herbicide glyphosate is a potent inhibitor of 5-enolpyruvyl-shikimic acid-3-phosphate synthase". Biochem. Biophys. Res. Commun. 94 (4): 1207–12. doi:10.1016/0006-291X(80)90547-1. PMID 7396959.
- ^ Purdue University, Department of Horticulture and Landscape Architecture, Metabolic Plant Physiology Lecture notes, Aromatic amino acid biosynthesis, The shikimate pathway – synthesis of chorismate.
- ^ Schönbrunn E, Eschenburg S, Shuttleworth WA, Schloss JV, Amrhein N, Evans JN, Kabsch W (February 2001). "Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail". Proc. Natl. Acad. Sci. U.S.A. 98 (4): 1376–80. Bibcode:2001PNAS...98.1376S. doi:10.1073/pnas.98.4.1376. PMC 29264. PMID 11171958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Glyphosate bound to proteins in the Protein Data Bank
- ^ Funke T, Han H, Healy-Fried ML, Fischer M, Schönbrunn E (August 2006). "Molecular basis for the herbicide resistance of Roundup Ready crops". Proc. Natl. Acad. Sci. U.S.A. 103 (35): 13010–5. Bibcode:2006PNAS..10313010. doi:10.1073/pnas.0603638103. JSTOR 30050705. PMC 1559744. PMID 16916934.
{{cite journal}}: Check|bibcode=length (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g h i j "Registration Decision Fact Sheet for Glyphosate (EPA-738-F-93-011)" (PDF). R.E.D. FACTS. United States Environmental Protection Agency. 1993.
- ^ a b c d e f National Pesticide Information Center Technical Factsheet on: GLYPHOSATE
- ^ Stevan Z. Knezevic, University of Nebraska Extension Integrated Weed Management Specialist, last revised Revised February 2010 Use of Herbicide-Tolerant Crops as Part of an Integrated Weed Management Program
- ^ Thomas M Amrein (2012-12-21). "Analysis of pesticides in food" (PDF). ETH Zurich. p. 15. Retrieved 06/02/2013.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Luijendijk CD et al Measures to reduce glyphosate runoff from hard surfaces Plant Research International B.V., Wageningen May 2005
- ^ Botta F, Lavison G, Couturier G, Alliot F, Moreau-Guigon E, Fauchon N, Guery B, Chevreuil M, Blanchoud H (September 2009). "Transfer of glyphosate and its degradate AMPA to surface waters through urban sewerage systems". Chemosphere. 77 (1): 133–9. doi:10.1016/j.chemosphere.2009.05.008. PMID 19482331.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b "The agronomic benefits of glyphosate in Europe" (PDF). Monsanto Europe SA. February 2010. Retrieved 06/02/2013.
{{cite web}}: Check date values in:|accessdate=(help) - ^ Lousisana State University Agricultural Extension Office. Last Updated: 3 September 2014 Sugarcane Ripener Recommendations - Glyphosate Page Accessed 3 September 2014
- ^ Farm Chemicals International Glyphosate entry in Crop Protection Database
- ^ Mitchem W. "Mirror or Mirror on the Wall Show Me the Best Glyphosate Formulation of All" (PDF). North Carolina State University Extension. Retrieved 2013-07-23.
- ^ Hartzler B. "ISU Weed Science Online - Glyphosate - A Review". Iowa State University Extension.
- ^ a b c Tu M, Hurd C, Robison R, Randall JM (2001-11-01). "Glyphosate" (PDF). Weed Control Methods Handbook. The Nature Conservancy.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ China Research & Intelligence, June 5, 2013. Research Report on Global and China Glyphosate Industry, 2013-2017
- ^ a b VanGessel M. "Glyphosate Formulations". Control Methods Handbook, Chapter 8, Adjuvants: Weekly Crop Update. University of Delaware Cooperative Extension.
- ^ Tu M, Randall JM (2003-06-01). "Glyphosate" (PDF). Weed Control Methods Handbook. The Nature Conservancy.
- ^ Curran WS, McGlamery MD, Liebl RA, Lingenfelter DD (1999). "Adjuvants for Enhancing Herbicide Performance". Penn State Extension.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Sprague C, Hager A (2000-05-12). "Principles of Postemergence Herbicides". University of Illinois Extension Service. Retrieved 2012-11-29.
- ^ Young B. "Adjuvant Products by Manufacturer, Compendium of Herbicide Adjuvants". Southern Illinois University.
- ^ e-phy: Le catalogue des produits phytopharmaceutiques et de leurs usages des matières fertilisantes et des supports de culture homologués en France
- ^ "The debate over whether Monsanto is a corporate sinner or saint". The Economist. 19 November 2009. Retrieved 20 November 2009.
- ^ Cavallaro M (2009-06-26). "The Seeds Of A Monsanto Short Play". Forbes. Retrieved 2009-07-11.
- ^ a b c d Bradberry SM, Proudfoot AT, Vale JA (2004). "Glyphosate poisoning". Toxicol Rev. 23 (3): 159–67. doi:10.2165/00139709-200423030-00003. PMID 15862083.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Sample label – Roundup Pro" (PDF). Armed Forces Pest Management Board.
- ^ Sribanditmongkol P, Jutavijittum P, Pongraveevongsa P, Wunnapuk K, Durongkadech P (September 2012). "Pathological and toxicological findings in glyphosate-surfactant herbicide fatality: a case report". Am J Forensic Med Pathol. 33 (3): 234–7. doi:10.1097/PAF.0b013e31824b936c. PMID 22835958.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mink PJ, Mandel JS, Lundin JI, Sceurman BK (November 2011). "Epidemiologic studies of glyphosate and non-cancer health outcomes: a review". Regul. Toxicol. Pharmacol. 61 (2): 172–84. doi:10.1016/j.yrtph.2011.07.006. PMID 21798302.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mink PJ, Mandel JS, Sceurman BK, Lundin JI (August 2012). "Epidemiologic studies of glyphosate and cancer: a review". Regul. Toxicol. Pharmacol. 63 (3): 440–52. doi:10.1016/j.yrtph.2012.05.012. PMID 22683395.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Williams AL, Watson RE, DeSesso JM (2012). "Developmental and reproductive outcomes in humans and animals after glyphosate exposure: a critical analysis". J Toxicol Environ Health B Crit Rev. 15 (1): 39–96. doi:10.1080/10937404.2012.632361. PMID 22202229.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Kier, L.D.; Kirkland, D. J. (April 2013). "Review of genotoxicity studies of glyphosate and glyphosate-based formulations". Crit Rev Toxicol. ;43(4):: 283–315. doi:10.3109/10408444.2013.770820. Retrieved 2014-10-05.
{{cite journal}}: CS1 maint: extra punctuation (link) - ^ Kimmel GL, Kimmel CA, Williams AL, DeSesso JM (February 2013). "Evaluation of developmental toxicity studies of glyphosate with attention to cardiovascular development". Crit. Rev. Toxicol. 43 (2): 79–95. doi:10.3109/10408444.2012.749834. PMC 3581053. PMID 23286529.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mink PJ, et al. Epidemiologic studies of glyphosate and cancer: a review. Regul Toxicol Pharmacol. 2012 Aug;63(3):440-52. doi: 10.1016/j.yrtph.2012.05.012. PMID 22683395
- ^ Schinasi, Leah; Leon, Maria E. (Apr 23, 2014). "Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: a systematic review and meta-analysis". Int J Environ Res Public Health. 11 (4): 4449–527. doi:10.3390/ijerph110404449. PMC 4025008. PMID 24762670.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ . NIH NCI Surveillance, Epidemiology, and End Results Program. April 2014 SEER Stat Fact Sheets: Non-Hodgkin Lymphoma http://seer.cancer.gov/statfacts/html/nhl.html SEER Stat Fact Sheets: Non-Hodgkin Lymphoma. Retrieved February 26, 2015.
{{cite web}}: Check|url=value (help); Missing or empty|title=(help) - ^ "Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate". The Lancet Oncology. Elsevier Ltd. 20 March 2015. Retrieved 21 March 2015.
{{cite web}}: Cite uses deprecated parameter|authors=(help) - ^ Associated Press (20 March 2015). "Roundup Weed Killer Has Probable Carcinogen, U.N. Says". US News & World Report. Retrieved 21 March 2015.
- ^ a b c d Giesy JP, Dobson S, Solomon KR (2000). "Ecotoxicological Risk Assessment for Roundup® Herbicide". Reviews of Environmental Contamination and Toxicology. Reviews of Environmental Contamination and Toxicology. 167: 35–120. doi:10.1007/978-1-4612-1156-3_2. ISBN 978-0-387-95102-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Andréa MM, Peres TB, Luchini LC, Bazarin S, Papini S, Matallo MB, Savoy VLT (2003). "Influence of repeated applications of glyphosate on its persistence and soil bioactivity". Pesquisa Agropecuária Brasileira. 38 (11): 1329. doi:10.1590/S0100-204X2003001100012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Balthazor TM, Hallas LE (February 1986). "Glyphosate-degrading microorganisms from industrial activated sludge". Appl. Environ. Microbiol. 51 (2): 432–4. PMC 238888. PMID 16346999.
- ^ Cerdeira AL, Duke SO (January 2010). "Effects of glyphosate-resistant crop cultivation on soil and water quality". GM Crops. 1 (1): 16–24. doi:10.4161/gmcr.1.1.9404. PMID 21912208.
- ^ Torstensson NT, Lundgren LN, Stenström J (October 1989). "Influence of climatic and edaphic factors on persistence of glyphosate and 2,4-D in forest soils". Ecotoxicol. Environ. Saf. 18 (2): 230–9. doi:10.1016/0147-6513(89)90084-5. PMID 2806176.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Albers CN, Banta GT, Hansen PE, Jacobsen OS (October 2009). "The influence of organic matter on sorption and fate of glyphosate in soil--comparing different soils and humic substances". Environ. Pollut. 157 (10): 2865–70. doi:10.1016/j.envpol.2009.04.004. PMID 19447533.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ole K. Borggaard OK (2011). "Does phosphate affect soil sorption and degradation of glyphosate? - A review". Trends in Soil Science and Plant Nutrition. 2 (1): 17–27.
- ^ Doublet J, Mamy L, Barriuso E (October 2009). "Delayed degradation in soil of foliar herbicides glyphosate and sulcotrione previously absorbed by plants: consequences on herbicide fate and risk assessment". Chemosphere. 77 (4): 582–9. doi:10.1016/j.chemosphere.2009.06.044. PMID 19625069.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hassan SA, Bigler F, Bogenschütz H, Boller E, Brun J, Calis JNM, Chiverton P, Coremans-Pelseneer J, Duso C (1991). "Results of the fifth joint pesticide testing programme carried out by the IOBC/WPRS-Working Group 'Pesticides and beneficial organisms'". Entomophaga. 36: 55–67. doi:10.1007/BF02374636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Springett JA, Gray RAJ (1992). "Effect of repeated low doses of biocides on the earthworm Aporrectodea caliginosa in laboratory culture". Soil Biology and Biochemistry. 24 (12): 1739. doi:10.1016/0038-0717(92)90180-6.
- ^ Preston CM, Trofymow JA (1989). "Effects of glyphosate (Roundup) on biological activity of forest soils". Proceedings of the Carnation Creek Workshop: 122–40. ISBN 0-7726-0917-9.
- ^ Dick R, Lorenz N, Wojno M, Lane M (2010). Microbial dynamics in soils under long-term glyphosate tolerant cropping systems (PDF). 19th World Congress of Soil Science.
{{cite conference}}: CS1 maint: multiple names: authors list (link) - ^ Santos A, Flores M (1995). "Effects of glyphosate on nitrogen fixation of free-living heterotrophic bacteria". Letters in Applied Microbiology. 20 (6): 349–52. doi:10.1111/j.1472-765X.1995.tb01318.x.
- ^ Yamada, T; Kremer, RJ,; de Camargo e Castro, PR; Wood, BW (2009). "Glyphosate interactions with physiology, nutrition, and diseases of plants: Threat to agricultural sustainability?". European Journal of Agronomy. 31 (3). Elsevier: 111–113. doi:10.1016/j.eja.2009.07.004.
{{cite journal}}: CS1 maint: extra punctuation (link) CS1 maint: multiple names: authors list (link) - ^ Clair E, Linn L, Travert C, Amiel C, Séralini GE, Panoff JM (May 2012). "Effects of Roundup(®) and glyphosate on three food microorganisms: Geotrichum candidum, Lactococcus lactis subsp. cremoris and Lactobacillus delbrueckii subsp. bulgaricus ". Curr. Microbiol. 64 (5): 486–91. doi:10.1007/s00284-012-0098-3. PMID 22362186.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) | Agriculture | US EPA".
- ^ "Pesticide Registration Manual | Pesticide Registration | US EPA".
- ^ a b Gary L. Diamond and Patrick R. Durkin February 6, 1997, under contract from the United States Department of Agriculture. Effects of Surfactants on the Toxicitiy of Glyphosate, with Specific Reference to RODEO
- ^ Loveland Products. X-77 Spreader Label
- ^ Loveland Products. Material Safety Data Sheet - X-77 Spreader
- ^ Loveland Products. alt X-77 Spreader Label
- ^ Note: Prior versions were known as "X-77 Spreader".[31] This product itself had various versions: one included alkylphenol ethoxylate, alcohol ethoxylate, tall oil fatty acid, 2,2' dihydroxydiethyl ethyl, and dimethylpolysiloxane.[72][73] Another version included alkylarylpolyoxyethylene, alkylpolyoxyethylene, gatty acids, glycols and dimethylpolysiloxane.[74]
- ^ Loveland Products. Spreader 90 Label
- ^ Loveland Products. Spreader 90 Material Safety Data Sheet
- ^ Staff, Environment Canada. February 2013 Federal Environmental Quality Guidelines: Alcohol Ethoxylates
- ^ Williams GM, Kroes R, Munro IC (April 2000). "Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans". Regul. Toxicol. Pharmacol. 31 (2 Pt 1): 117–65. doi:10.1006/rtph.1999.1371. PMID 10854122.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Review report for the active substance glyphosate" (PDF). Commission working document. European Commission, Health and Protection Directorate-General: Directorate E – Food Safety: plant health, animal health and welfare, international questions: E1 - Plant Health. 2002-01-21.
- ^ Goldstein DA, Acquavella JF, Mannion RM, Farmer DR (2002). "An analysis of glyphosate data from the California Environmental Protection Agency Pesticide Illness Surveillance Program". J. Toxicol. Clin. Toxicol. 40 (7): 885–92. doi:10.1081/CLT-120016960. PMID 12507058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c "Pesticide Illness Surveillance Program". California Pesticide Illness Serveillance Program Report HS-1733. California EPA. 2010.
- ^ Talbot AR, Shiaw MH, Huang JS, Yang SF, Goo TS, Wang SH, Chen CL, Sanford TR (January 1991). "Acute poisoning with a glyphosate-surfactant herbicide ('Roundup'): a review of 93 cases". Hum Exp Toxicol. 10 (1): 1–8. doi:10.1177/096032719101000101. PMID 1673618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Romano RM, Romano MA, Bernardi MM, Furtado PV, Oliveira CA (April 2010). "Prepubertal exposure to commercial formulation of the herbicide glyphosate alters testosterone levels and testicular morphology". Arch. Toxicol. 84 (4): 309–17. doi:10.1007/s00204-009-0494-z. PMID 20012598.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ EPA Federal Register http://www.epa.gov/endo/pubs/draft_list_frn_061807.pdf
- ^ ToxNet. Glyposate. National Library of Medicine.
- ^ a b András Székács and Béla Darvas. Forty years with glyphosate. In: Herbicides - Properties, Synthesis and Control of Weeds", Ed. Mohammed Naguib Abd El-Ghany Hasaneen, ISBN 978-953-307-803-8, Published: January 13, 2012.
- ^ Peluso M, Munnia A, Bolognesi C, Parodi S (1998). "32P-postlabeling detection of DNA adducts in mice treated with the herbicide Roundup". Environ. Mol. Mutagen. 31 (1): 55–9. doi:10.1002/(SICI)1098-2280(1998)31:1<55::AID-EM8>3.0.CO;2-A. PMID 9464316.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Solomon KR, Thompson DG (2003). "Ecological risk assessment for aquatic organisms from over-water uses of glyphosate". J Toxicol Environ Health B Crit Rev. 6 (3): 289–324. doi:10.1080/10937400306468. PMID 12746143.
- ^ Wagner, N (Aug 2013). "Questions concerning the potential impact of glyphosate-based herbicides on amphibians". Environ Toxicol Chem. 32 (8): 1688–700. doi:10.1002/etc.2268. PMID 23637092.
- ^ Pesticide Action Network Asia & the Pacific (PANAP) Glyphosate 2009
- ^ Paganelli A, Gnazzo V, Acosta H, López SL, Carrasco AE (October 2010). "Glyphosate-based herbicides produce teratogenic effects on vertebrates by impairing retinoic acid signaling". Chem. Res. Toxicol. 23 (10): 1586–95. doi:10.1021/tx1001749. PMID 20695457.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Response to "The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities"" (PDF). Backgrounder. Monsanto Company. 2005-04-01.
- ^ "Aquatic Use of Glyphosate Herbicides in Australia" (PDF). Backgrounder. Monsanto Company. 2003-05-01.
- ^ Wojtaszek BF, Staznik B, Chartrand DT, Stephenson GR, Thompson DG (April 2004). "Effects of Vision® herbicide on mortality, avoidance response, and growth of amphibian larvae in two forest wetlands". Environ. Toxicol. Chem. 23 (4): 832–42. doi:10.1897/02-281. PMID 15095877.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fernandez MR, Selles F, Gehl D, Depauw RM, Zentner RP (2005). "Crop Production Factors Associated with Fusarium Head Blight in Spring Wheat in Eastern Saskatchewan". Crop Science. 45 (5): 1908–16. doi:10.2135/cropsci2004.0197.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Duke SO, Wedge DE, Cerdeira AL, Matallo MB (2007). "Interactions of Synthetic Herbicides with Plant Disease and Microbial Herbicides". In Vurro, Maurizio; Gressel, Jonathan (eds.). Novel Biotechnologies for Biocontrol Agent Enhancement and Management. NATO Security through Science Series. pp. 277–96. doi:10.1007/978-1-4020-5799-1_15. ISBN 978-1-4020-5797-7.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Lori (2009-05-07). "U of G Researchers Find Suspected Glyphosate-Resistant Weed". Uoguelph.ca. Retrieved 2010-08-22.
- ^ "Resisting Roundup". The New York Times. 2010-05-16.
- ^ Tarter S (2009-04-06). "PJStar.com". PJStar.com. Retrieved 2010-08-22.
- ^ ISU Weed Science Online – Are RR Weeds in Your Future I
- ^ Powles SB, Lorraine-Colwill DF, Dellow JJ, Preston C (1998). "Evolved Resistance to Glyphosate in Rigid Ryegrass (Lolium rigidum) in Australia". Weed Science. 46 (5): 604–7. JSTOR 4045968.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Glyphosate resistance is a reality that should scare some cotton growers into changing the way they do business". Southeastfarmpress.com. Retrieved 2010-08-22.
- ^ "Map of Glyphosate-Resistant Weeds Globally". The International Survey of Herbicide Resistant Weeds. 2010. Retrieved 12 Jan 2013.
- ^ Neuman W, Pollack A (4 May 2010). "U.S. Farmers Cope With Roundup-Resistant Weeds". New York Times. New York. pp. B1. Retrieved 4 May 2010.
- ^ Charles M Benbrook Impacts of genetically engineered crops on pesticide use in the U.S. - the first sixteen years Environmental Sciences Europe 2012, 24:24
- ^ Heap, I. The International Survey of Herbicide Resistant Weeds. Online. Accessed April 13, 2014 Resistance by Active Ingredient (select "glyphosate" from the pulldown menu)
- ^ Culpepper AS, Grey TL, Vencill WK, Kichler JM, Webster TM, Brown SM, York AC Davis JW, Hanna WW (2006). "Glyphosate-resistant Palmer amaranth (Amaranthus palmeri ) confirmed in Georgia". Weed Science. 54 (4): 620–6. doi:10.1614/WS-06-001R.1. JSTOR 4539441.
{{cite journal}}: hair space character in|title=at position 57 (help)CS1 maint: multiple names: authors list (link) - ^ a b Hampton N. "Cotton versus the monster weed". Retrieved 2009-07-19.
- ^ a b Smith JT (March 2009). "Resistance a growing problem" (PDF). The Farmer Stockman. Retrieved 2009-07-19.
- ^ Taylor O (2009-07-16). "Peanuts: variable insects, variable weather, Roundup resistant Palmer in new state". PeanutFax. AgFax Media. Retrieved 2009-07-19.
- ^ Vargas L, Bianchi MA, Rizzardi MA, Agostinetto D, Dal Magro T (2007). "Buva (Conyza bonariensis) resistente ao glyphosate na região sul do Brasil". Planta Daninha (in Portuguese). 25 (3): 573–8. doi:10.1590/S0100-83582007000300017.
{{cite journal}}: Unknown parameter|trans_title=ignored (|trans-title=suggested) (help)CS1 maint: multiple names: authors list (link) - ^ Koger CH, Shaner DL, Henry WB, Nadler-Hassar T, Thomas WE, Wilcut JW (2005). "Assessment of two nondestructive assays for detecting glyphosate resistance in horseweed (Conyza canadensis)". Weed Science. 53 (4): 438–45. doi:10.1614/WS-05-010R. JSTOR 4047050.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ge X, d'Avignon DA, Ackerman JJ, Sammons RD (April 2010). "Rapid vacuolar sequestration: the horseweed glyphosate resistance mechanism". Pest Manag. Sci. 66 (4): 345–8. doi:10.1002/ps.1911. PMC 3080097. PMID 20063320.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vila-Aiub MM, Vidal RA, Balbi MC, Gundel PE, Trucco F, Ghersa CM (April 2008). "Glyphosate-resistant weeds of South American cropping systems: an overview". Pest Manag. Sci. 64 (4): 366–71. doi:10.1002/ps.1488. PMID 18161884.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Preston C, Wakelin AM, Dolman FC, Bostamam Y, Boutsalis P (2009). "A Decade of Glyphosate-Resistant Lolium around the World: Mechanisms, Genes, Fitness, and Agronomic Management". Weed Science. 57 (4): 435–41. doi:10.1614/WS-08-181.1.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vila-Aiub MM, Balbi MC, Gundel PE, Ghersa CM, Powles SB (2007). "Evolution of Glyphosate-Resistant Johnsongrass (Sorghum halepense) in Glyphosate-Resistant Soybean". Weed Science. 55 (6): 566–71. doi:10.1614/WS-07-053.1. JSTOR 4539618.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Charry T (1997-05-29). "Monsanto recruits the horticulturist of the San Diego Zoo to pitch its popular herbicide". Business Day. New York Times.
- ^ a b "Testing Fraud: IBT and Craven Labs" (PDF). Backgrounder. Monsanto Company. June 2005.
- ^ Schneider K (Spring 1983). "Faking it The Case against Industrial Bio-Test Laboratories". The Amicus Journal. PlanetWaves.net: 14–26.
- ^ "EPA FY1994 Enforcement and Compliance Assurance Accomplishments Report" (PDF). United States Environmental Protection Agency.
- ^ Piller D (2010-04-01). "Albaugh accuses Chinese of dumping herbicide". Staff Blogs. Des Moines Register.
- ^ "In the Matter of: GLYPHOSATE FROM CHINA" (PDF). United States International Trade Commission. 2010-04-22.
- ^ Green JM, Owen MD (2011). "Herbicide-resistant crops: utilities and limitations for herbicide-resistant weed management". J. Agric. Food Chem. 59 (11): 5819–29. doi:10.1021/jf101286h. PMC 3105486. PMID 20586458.
- ^ Pollegioni L, Schonbrunn E, Siehl D (2011). "Molecular basis of glyphosate resistance-different approaches through protein engineering". FEBS J. 278 (16): 2753–66. doi:10.1111/j.1742-4658.2011.08214.x. PMC 3145815. PMID 21668647.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rashid A (2009). Introduction to Genetic Engineering of Crop Plants: Aims and Achievements. I K International. p. 259. ISBN 978-93-80026-16-9.
- ^ "Company History". Web Site. Monsanto Company.
- ^ Hamer H (2010-06-30). "Acreage" (PDF). National Agricultural Statistics Board Annual Report,. United States Department of Agriculture.
{{cite web}}: CS1 maint: extra punctuation (link)
