Hepatitis C
| Hepatitis C | |
|---|---|
| Specialty | Infectious diseases |
| Frequency | 22% (Egypt), 48% (Pakistan), 32% (People's Republic of China) |
Hepatitis C is an infectious disease affecting primarily the liver, caused by the hepatitis C virus (HCV).[1] The infection is often asymptomatic, but chronic infection can lead to scarring of the liver and ultimately to cirrhosis, which is generally apparent after many years. In some cases, those with cirrhosis will go on to develop liver failure, liver cancer or life-threatening esophageal and gastric varices.[1]
The hepatitis C virus is spread primarily by blood-to-blood contact associated with intravenous drug use, poorly sterilized medical equipment and transfusions. An estimated 130–170 million people worldwide are infected with hepatitis C. The existence of hepatitis C (originally "non-A non-B hepatitis") was postulated in the 1970s and proven in 1989.[2] It is not known to cause disease in other animals.
The virus persists in the liver in about 85% of those infected. This persistent infection can be treated with medication; peginterferon and ribavirin are the current standard therapy. Overall, between 50–80% of people treated are cured. Those who develop cirrhosis or liver cancer may require a liver transplant. Hepatitis C is the leading cause of liver transplantation though the virus usually recurs after transplantation.[3] No vaccine against hepatitis C is currently available.
Signs and symptoms
Acute infection
Hepatitis C infection causes acute symptoms in 15% of cases.[4] Symptoms are generally mild and vague, including a decreased appetite, fatigue, nausea, muscle or joint pains, and weight loss.[5] Most cases of acute infection are not associated with jaundice.[6] The infection resolves spontaneously in 10-50% of cases, which occurs more frequently in individuals who are young and female.[6]
Chronic infection
About 80% of those exposed to the virus develop a chronic infection.[7] Most experience minimal or no symptoms during the initial few decades of the infection,[8] although chronic hepatitis C can be associated with fatigue.[9] Hepatitis C after many years becomes the primary cause of cirrhosis, and liver cancer.[3] About 10–30% of people develop cirrhosis over 30 years.[5][3] Cirrhosis is more common in those co infected with hepatitis B or HIV, alcoholics, and those of male gender.[5] Those who develop cirrhosis have a 20 fold greater risk of hepatocellular carcinoma, a rate of 1-3% per year,[5][3] and if this is complicated by excess alcohol the risk becomes 100 fold greater.[10] Hepatitis C is the cause of 27% of cirrhosis cases and 25% of hepatocellular carcinoma worldwide.[11]
Liver cirrhosis may lead to portal hypertension, ascites (accumulation of fluid in the abdomen), easy bruising or bleeding, varices (enlarged veins, especially in the stomach and esophagus), jaundice, and a syndrome of cognitive impairment known as hepatic encephalopathy. It is a common cause for requiring a liver transplant.[12]
Extrahepatic
Hepatitis C is also rarely associated with Sjögren's syndrome (an autoimmune disorder), thrombocytopenia, lichen planus, diabetes mellitus, and B-cell lymphoproliferative disorders.[13] Thrombocytopenia is estimated to occur in 0.16% to 45.4% of people with chronic hepatitis C.[14] Putative associations with Hyde's prurigo nodularis [15] and membranoproliferative glomerulonephritis have been reported.[9]
Virology
The hepatitis C virus (HCV) is a small, enveloped, single-stranded, positive-sense RNA virus.[3] It is a member of the hepacivirus genus in the family Flaviviridae.[9] There are seven major genotypes of HCV, which are indicated numerically from one to seven.[16] In the United States, about 70% of cases are caused by genotype 1, 20% by genotype 2, and about 1% by each of the other genotypes.[5] Genotype 1 is also the most common in South America and Europe.[3]
Transmission
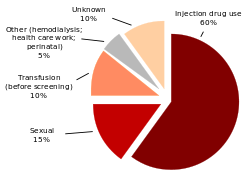
The primary methods of transmission in the developed world is intravenous drug use (IDU), while in the developing world the main methods are blood transfusions and unsafe medical procedures.[17] The cause of transmission remains unknown in 20% of cases;[18] however, many of these are believed to be accounted for by IDU.[6]
Intravenous drug use
IDU is a major risk factor for hepatitis C in many parts of the world.[19] Of a review of 77 countries 25 had rates of hepatitis C in the intravenous drug user population of between 60% and 80% including the United States[7] and China.[19] While twelve countries had rates greater than 80%.[7] It is believed that ten million intravenous drug users are infected with hepatitis C; China (1.6 million), the United States (1.5 million), and Russia (1.3 million) have the highest absolute totals.[7] Rates of hepatitis C among prison inmates in the United States are ten to 20 times that of the rates of the general population, which is attributed to high-risk behavior such as IDU and tattooing with nonsterile equipment.[20][21]
Healthcare exposure
Blood transfusion, blood products, or organ transplantation without HCV screening is a significant risk for infection.[5] The United States instituted universal screening in 1992 and the risk subsequently has decreased to one in 10,000 to 10,000,000 per units of blood[18][6] down from a risk of one in 200 units of blood.[22] This low risk remains as there is a period of about 11–70 days between the potential blood donor acquiring hepatitis C and their blood testing positive depending on the method.[18] Some countries still do not screen for hepatitis C due to the cost.[11]
Those who have experienced a needle stick injury from someone who was HCV positive have about a 1.8% chance of subsequently contacting the disease themselves.[5] The risk is greater if the needle in question is hollow and the puncture wound is deep.[11] There is a risk from mucosal exposures to blood; but this risk is low, and there is no risk if blood exposure occurs on intact skin.[11]
Hospital equipment has also been documented as a method of transmission of hepatitis C including: reuse of needles and syringes, multiple-use medication vials, infusion bags, and improperly sterilized surgical equipment, among others.[11] Limitations in the implementation and enforcement of stringent standard precautions in public and private medical and dental facilities are known to be the primary cause of the spread of HCV in Egypt, the country with highest rate of infection in the world.[23]
Sexual intercourse
Whether hepatitis C can be transmitted through sexual activity is controversial.[24] While there is an association between high-risk sexual activity and hepatitis C, it is not known whether transmission of the disease is due to drug use that has not been admitted to or sex as a risk factor.[5] The majority of evidence supports there being no risk for monogamous heterosexual couples.[24] Sexual practices that involve higher levels of trauma to the anogenital mucosa, such as anal penetrative sex, or that occur when there is a concurrent sexually transmitted infection, including HIV or genital ulceration, do present a risk.[24] The United States government only recommends condom use to prevent hepatitis C transmission in those with multiple partners.[25]
Body piercings
Tattooing is associated with two to three fold increased risk of hepatitis C.[26] This can be due to either improperly sterilized equipment or contamination of the dyes being used.[26] Tattoos or piercings performed either before the mid-1980s, "underground," or nonprofessionally are of particular concern, since sterile techniques in such settings may be lacking. The risk also appears to be greater for larger tattoos.[26] It is estimated that nearly half of prison inmates share unsterilized tattooing equipment.[26] It is rare for tattoos in a licensed facility to be directly associated with HCV infection.[27]
Personal-care items such as razors, toothbrushes, and manicuring or pedicuring equipment can be contaminated with blood. Sharing such items can potentially lead to exposure to HCV.[28][29] Appropriate caution should be taken regarding any medical condition that results in bleeding, such as cuts and sores.[29] HCV is not spread through casual contact, such as hugging, kissing, or sharing eating or cooking utensils.[29]
Vertical transmission
Vertical transmission of hepatitis C from an infected mother to her child occurs in less than 10% of pregnancies.[30] There are no measures that alter this risk.[30] It is not clear when during pregnancy transmission occurs, but it may occur both during gestation and at delivery.[18] A long labor is associated with a greater risk of transmission.[11] There is no evidence that breast-feeding spreads HCV; however, to be cautious, an infected mother is advised to avoid breastfeeding if her nipples are cracked and bleeding,[31] or her viral loads are high.[18]
Diagnosis
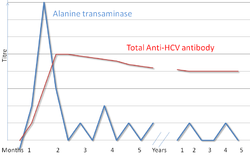
There are a number of diagnostic tests for hepatitis C including: HCV antibody enzyme immunoassay or ELISA, recombinant immunoblot assay, and quantitative HCV RNA polymerase chain reaction (PCR).[5] HCV RNA can be detected by PCR typically one to two weeks after infection, while antibodies can take substantially longer to form and thus be detected.[12]
Chronic hepatitis C is defined as infection with the hepatitis C virus persisting for more than six months based on the presence of its RNA.[8] Chronic infections are typically without symptomatic during the first few decades,[8] and thus it is most commonly discovered following the investigation of elevated liver enzyme levels or during a routine screening of high risk individuals. Testing is not able to distinguish between acute and chronic infections.[11]
Serology
Hepatitis C testing typically begins with blood testing to detect the presence of antibodies to the HCV using an enzyme immunoassay.[5] If this test is positive, a confirmatory test is then performed to verify the immunoassay and to determine the viral load.[5] A recombinant immunoblot assay is used to verify the immunoassay and the viral load is determined by a HCV RNA polymerase chain reaction.[5] If there are no RNA and the immunoblot is positive it means that the person had a previous infection but cleared it either with treatment or spontaneously; if the immunoblot is negative, it means that the immunoassay was wrong.[5] It takes about 6-8 weeks following infection before the immunoassay will test positive.[9]
Liver enzymes are variable during the initial part of the infection[8] and on average begin to rise at seven weeks after infection.[9] Liver enzymes are poorly related with disease severity.[9]
Biopsy
Liver biopsies are used to determine the degree of liver damage present; however, there are risks from the procedure.[3] The typical changes seen are lymphocytes within the parenchyma, lymphoid follicles in portal triad, and changes to the bile ducts.[3] There are a number of blood tests available that try to determine the degree of hepatic fibrosis and alleviate the need for biopsy.[3]
Screening
It is believed only 5–50% of those infected in the United States and Canada become aware of their status.[26] Testing is recommended in those at high risk, which includes those with tattoos.[26] Screening is also recommended in those with elevated liver enzymes as this is frequently the only sign of chronic hepatitis.[32] Routine screening, however, is not recommended in the United States.[5]
Prevention
As of 2011, no vaccine protects against contracting hepatitis C. However, a number are under development and some have shown encouraging results.[33] A combination of harm reduction strategies, such as the provision of new needles and syringes and treatment of substance use, decrease the risk of hepatitis C in intravenous drug users by about 75%.[34] The screening of blood donors is important at a national level, as is adhering to universal precautions within healthcare facilities.[9] In countries where there is an insufficient supply of sterile syringes, medications should be given orally rather than via injection.[11]
Treatment
HCV induces chronic infection in 50–80% of infected persons. Approximately 40-80% of these clear with treatment.[35][36] In rare cases, infection can clear without treatment.[6] Those with chronic hepatitis C are advised to avoid alcohol and medications toxic to the liver,[5] and to be vaccinated for hepatitis A and hepatitis B.[5] Ultrasound surveillance for hepatocellular carcinoma is recommended in those with accompanying cirrhosis.[5]
Medications
In general, treatment is recommended in those with proven HCV infection liver abnormalities.[5] Current treatment is a combination pegylated interferon alpha and the antiviral drug ribavirin for a period of 24 or 48 weeks, depending on HCV genotype.[5] When combined with ribavirin, pegylated interferon-alpha-2a may be superior to pegylated interferon-alpha-2b, though the evidence is not strong.[37] Improved outcomes are seen in 50–60% of people.[5] Combining either boceprevir or telaprevir with ribavirin and peginterferon alfa improves antiviral response for hepatitis C genotype 1.[38][39][40] Adverse effect with treatment are common with half of people getting flu like symptoms and a third experiencing emotional problems.[5] Treatment during the first six month is more effective than once hepatitis C has become chronic.[12] If someone develops and new infection and it has not cleared after eight to twelve weeks, 24 weeks of pegylated interferon is recommended.[12] In people with thalassemia, ribavirin appears to be useful but increases the need for transfusions.[41]
Alternative medicine
Several alternative therapies are claimed by their proponents to be helpful for hepatitis C including milk thistle, ginseng, and colloidal silver.[42] However, no alternative therapy has been shown to improve outcomes in hepatitis C.[42][43][44]
Prognosis
Responses to treatment vary by genotype. Sustained response is about 40-50% in people with HCV genotype 1 given 48 weeks of treatment.[3] Sustained response is seen in 70-80% of people with HCV genotypes 2 and 3 with 24 weeks of treatment.[3] Sustained response is about 65% in those with genotype 4 given 48 weeks of treatment. The evidence for treatment in genotype 6 disease is currently sparse, and the evidence that exists is for 48 weeks of treatment at the same doses as are used for genotype 1 disease.[45]
Epidemiology

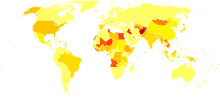
It is estimated that 130–170 million people, or ~3% of the world's population, are living with chronic hepatitis C.[46] About 3–4 million people are infected per year, and more than 350,000 people die yearly from hepatitis C-related diseases.[46] Rates have increased substantially in the 20th century due to a combination of IDU and intravenous medication or poorly sterilized medical equipment.[47]
In the United States, about 2% of people have hepatitis C,[5] with about 35,000 to 185,000 new cases a year. Rates have decreased in the Western world since the 1990s due to improved screening of blood before transfusion.[12] Annual deaths from HCV in the United States range from 8,000 to 10,000; expectations are that this mortality rate will increase, as those infected by transfusion before HCV testing become apparent.[48]
Prevalence is higher in some countries in Africa and Asia.[49] Countries with particularly high rates of infection include Egypt (22%), Pakistan (4.8%) and China (3.2%).[46] It is believed that the high prevalence in Egypt is linked to a now-discontinued mass-treatment campaign for schistosomiasis, using improperly sterilized glass syringes.[11]
History
In the mid-1970s, Harvey J. Alter, Chief of the Infectious Disease Section in the Department of Transfusion Medicine at the National Institutes of Health, and his research team demonstrated how most post-transfusion hepatitis cases were not due to hepatitis A or B viruses. Despite this discovery, international research efforts to identify the virus, initially called non-A, non-B hepatitis (NANBH), failed for the next decade. In 1987, Michael Houghton, Qui-Lim Choo, and George Kuo at Chiron Corporation, collaborating with Dr. D.W. Bradley from Centers for Disease Control and Prevention, used a novel molecular cloning approach to identify the unknown organism and develop a diagnostic test.[50] In 1988, the virus was confirmed by Alter by verifying its presence in a panel of NANBH specimens. In April 1989, the discovery of HCV was published in two articles in the journal Science.[51][52] The discovery led to significant improvements in diagnosis and improved antiviral treatment.[50] In 2000, Drs. Alter and Houghton were honored with the Lasker Award for Clinical Medical Research for "pioneering work leading to the discovery of the virus that causes hepatitis C and the development of screening methods that reduced the risk of blood transfusion-associated hepatitis in the U.S. from 30% in 1970 to virtually zero in 2000."[53]
Chiron filed for several patents on the virus and its diagnosis.[54] A competing patent application by the CDC was dropped in 1990 after Chiron paid $1.9 million to the CDC and $337,500 to Bradley. In 1994, Bradley sued Chiron, seeking to invalidate the patent, have himself included as a coinventor, and receive damages and royalty income. He dropped the suit in 1998 after losing before an appeals court.[55]
Society and culture
World Hepatitis Day, held on July 28, is coordinated by the World Hepatitis Alliance.[56] The economic cost of hepatitis C are significant both to the individual and to society. In the United States the average lifetime cost of the disease was estimated at 33,407 USD in 2003[57] with the cost of a liver transplant as of 2011 costing approximately 200,000 USD.[58] In Canada the cost of a course of antiviral treatment is as high as 30,000 CAD in 2003,[59] while is the United States costs are between 9,200 and 17,600 in 1998 USD.[57] In many areas of the world people are unable to afford treatment with antivirals as they either lack insurance coverage or the insurance they have will not pay for antivirals.[60]
Research
As of 2011, there are about one hundred medications in development for hepatitis C.[58] These include vaccines to treat hepatitis, immunomodulators, and cyclophilin inhibitors, among others.[61] These potentially new treatments have come about due to a better understanding of the hepatitis C virus.[62]
References
- ^ a b Ryan KJ, Ray CG (editors), ed. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 551–2. ISBN 0838585299.
{{cite book}}:|editor=has generic name (help) - ^ Houghton M (2009). "The long and winding road leading to the identification of the hepatitis C virus". Journal of Hepatology. 51 (5): 939–48. doi:10.1016/j.jhep.2009.08.004. PMID 19781804.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c d e f g h i j k Rosen, HR (2011 Jun 23). "Clinical practice. Chronic hepatitis C infection" (PDF). The New England journal of medicine. 364 (25): 2429–38. PMID 21696309.
{{cite journal}}: Check date values in:|date=(help) - ^ Maheshwari, A (2008 Jul 26). "Acute hepatitis C.". Lancet. 372 (9635): 321–32. PMID 18657711.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i j k l m n o p q r s t u v Wilkins, T (2010 Jun 1). "Hepatitis C: diagnosis and treatment". American family physician. 81 (11): 1351–7. PMID 20521755.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e Chronic Hepatitis C Virus Advances in Treatment, Promise for the Future. Springer Verlag. 2011. p. 4. ISBN 9781461411918.
- ^ a b c d Nelson, PK (2011 Aug 13). "Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews". Lancet. 378 (9791): 571–83. PMID 21802134.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d Chronic Hepatitis C Virus Advances in Treatment, Promise for the Future. Springer Verlag. 2011. pp. 103–104. ISBN 9781461411918.
- ^ a b c d e f g Dolin, [edited by] Gerald L. Mandell, John E. Bennett, Raphael (2010). Mandell, Douglas, and Bennett's principles and practice of infectious diseases (7th ed. ed.). Philadelphia, PA: Churchill Livingstone/Elsevier. pp. Chapter 154. ISBN 978-0443068393.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Mueller, S (2009 Jul 28). "Alcoholic liver disease and hepatitis C: a frequently underestimated combination". World journal of gastroenterology : WJG. 15 (28): 3462–71. PMID 19630099.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h i Alter, MJ (2007 May 7). "Epidemiology of hepatitis C virus infection". World journal of gastroenterology : WJG. 13 (17): 2436–41. PMID 17552026.
{{cite journal}}: Check date values in:|date=(help) - ^ a b c d e Ozaras, R (2009 Apr). "Acute hepatitis C: prevention and treatment". Expert review of anti-infective therapy. 7 (3): 351–61. PMID 19344247.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Zignego AL, Ferri C, Pileri SA, Caini P, Bianchi FB (2007). "Extrahepatic manifestations of Hepatitis C Virus infection: a general overview and guidelines for a clinical approach". Digestive and Liver Disease. 39 (1): 2–17. doi:10.1016/j.dld.2006.06.008. PMID 16884964.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Louie, KS (2011 Jan). "Prevalence of thrombocytopenia among patients with chronic hepatitis C: a systematic review". Journal of viral hepatitis. 18 (1): 1–7. PMID 20796208.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Lee, MR (2005 Nov). "Prurigo nodularis: a review". The Australasian journal of dermatology. 46 (4): 211–18, quiz 219-20. PMID 16197418.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Nakano T, Lau GM, Lau GM, Sugiyama M, Mizokami M (2011). "An updated analysis of hepatitis C virus genotypes and subtypes based on the complete coding region". Liver Int. doi:10.1111/j.1478-3231.2011.02684.x. PMID 22142261.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Maheshwari, A (2010 Feb). "Management of acute hepatitis C.". Clinics in liver disease. 14 (1): 169–76, x. PMID 20123448.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e Pondé, RA (2011 Feb). "Hidden hazards of HCV transmission". Medical microbiology and immunology. 200 (1): 7–11. PMID 20461405.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Xia, X (2008 Oct). "Epidemiology of HCV infection among injection drug users in China: systematic review and meta-analysis". Public health. 122 (10): 990–1003. PMID 18486955.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Imperial, JC (2010 Jun). "Chronic hepatitis C in the state prison system: insights into the problems and possible solutions". Expert review of gastroenterology & hepatology. 4 (3): 355–64. PMID 20528122.
{{cite journal}}: Check date values in:|date=(help) - ^ Vescio, MF (2008 Apr). "Correlates of hepatitis C virus seropositivity in prison inmates: a meta-analysis". Journal of epidemiology and community health. 62 (4): 305–13. PMID 18339822.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Marx, John (2010). Rosen's emergency medicine: concepts and clinical practice 7th edition. Philadelphia, PA: Mosby/Elsevier. p. 1154. ISBN 9780323054720.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ "Highest Rates of Hepatitis C Virus Transmission Found in Egypt". Al Bawaba. 2010-08-09. Retrieved 2010-08-27.
- ^ a b c Tohme RA, Holmberg SD (2010). "Is sexual contact a major mode of hepatitis C virus transmission?". Hepatology. 52 (4): 1497–505. doi:10.1002/hep.23808. PMID 20635398.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ "Hepatitis C Group Education Class". United States Department of Veteran Affairs.
- ^ a b c d e f Jafari, S (2010 Nov). "Tattooing and the risk of transmission of hepatitis C: a systematic review and meta-analysis" (PDF). International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 14 (11): e928-40. PMID 20678951.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Hepatitis C" (PDF). Center for Disease Control and Prevention. Retrieved 2 January 2012.
- ^ Lock G, Dirscherl M, Obermeier F; et al. (2006). "Hepatitis C — contamination of toothbrushes: myth or reality?". J. Viral Hepat. 13 (9): 571–3. doi:10.1111/j.1365-2893.2006.00735.x. PMID 16907842.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c "Hepatitis C". FAQ – CDC Viral Hepatitis. Retrieved 2 Jan 2012.
- ^ a b Lam, NC (2010 Nov 15). "Caring for pregnant women and newborns with hepatitis B or C.". American family physician. 82 (10): 1225–9. PMID 21121533.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Mast EE (2004). "Mother-to-infant hepatitis C virus transmission and breastfeeding". Advances in Experimental Medicine and Biology. 554: 211–6. PMID 15384578.
- ^ Senadhi, V (2011 Jul). "A paradigm shift in the outpatient approach to liver function tests". Southern medical journal. 104 (7): 521–5. PMID 21886053.
{{cite journal}}: Check date values in:|date=(help) - ^ Halliday, J (2011 May). "Vaccination for hepatitis C virus: closing in on an evasive target". Expert review of vaccines. 10 (5): 659–72. PMID 21604986.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Hagan, H (2011 Jul 1). "A systematic review and meta-analysis of interventions to prevent hepatitis C virus infection in people who inject drugs". The Journal of infectious diseases. 204 (1): 74–83. PMID 21628661.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Torresi, J (2011 Jun). "Progress in the development of preventive and therapeutic vaccines for hepatitis C virus". Journal of hepatology. 54 (6): 1273–85. PMID 21236312.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Ilyas, JA (2011 Aug). "An overview of emerging therapies for the treatment of chronic hepatitis C.". Clinics in liver disease. 15 (3): 515–36. PMID 21867934.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C (2010). "Peginterferon alpha-2a is associated with higher sustained virological response than peginterferon alfa-2b in chronic hepatitis C: systematic review of randomized trials". Hepatology. 51 (4): 1176–84. doi:10.1002/hep.23504. PMID 20187106.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Foote BS, Spooner LM, Belliveau PP (2011). "Boceprevir: a protease inhibitor for the treatment of chronic hepatitis C". Ann Pharmacother. 45 (9): 1085–93. doi:10.1345/aph.1P744. PMID 21828346.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Smith LS, Nelson M, Naik S, Woten J (2011). "Telaprevir: an NS3/4A protease inhibitor for the treatment of chronic hepatitis C". Ann Pharmacother. 45 (5): 639–48. doi:10.1345/aph.1P430. PMID 21558488.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB (2011). "An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases". Hepatology. 54 (4): 1433–44. doi:10.1002/hep.24641. PMC 3229841. PMID 21898493.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Alavian SM, Tabatabaei SV (2010). "Treatment of chronic hepatitis C in polytransfused thalassaemic patients: a meta-analysis". J. Viral Hepat. 17 (4): 236–44. doi:10.1111/j.1365-2893.2009.01170.x. PMID 19638104.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Hepatitis C and CAM: What the Science Says. NCCAM March 2011. (Retrieved 07 March 2011)
- ^ Liu, J (2003 Mar). "Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials". The American journal of gastroenterology. 98 (3): 538–44. PMID 12650784.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Rambaldi, A (2007 Oct 17). "Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases". Cochrane database of systematic reviews (Online) (4): CD003620. PMID 17943794.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Fung J, Lai CL, Hung I; et al. (2008). "Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin". The Journal of Infectious Diseases. 198 (6): 808–12. doi:10.1086/591252. PMID 18657036.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c "WHO Hepatitis C factsheet". 2011. Retrieved 2011-07-13.
- ^ Alter, MJ (2007 May 7). "Epidemiology of hepatitis C virus infection". World journal of gastroenterology : WJG. 13 (17): 2436–41. PMID 17552026.
{{cite journal}}: Check date values in:|date=(help) - ^ Colacino, ed. by J. M. (2004). Hepatitis prevention and treatment. Basel: Birkhäuser. p. 32. ISBN 9783764359560.
{{cite book}}:|first=has generic name (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ al.], edited by Gary W. Brunette ... [et. CDC health information for international travel : the Yellow Book 2012. New York: Oxford University. p. 231. ISBN 9780199769018.
{{cite book}}:|first=has generic name (help) - ^ a b Boyer, JL (2001). Liver cirrhosis and its development: proceedings of the Falk Symposium 115. Springer. pp. 344. ISBN 9780792387602.
- ^ Choo QL, Kuo G, Weiner AJ, Overby LR, Bradley DW, Houghton M (1989). "Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome". Science. 244 (4902): 359–62. doi:10.1126/science.2523562. PMID 2523562.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kuo G, Choo QL, Alter HJ; et al. (1989). "An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis". Science. 244 (4902): 362–4. doi:10.1126/science.2496467. PMID 2496467.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Winners Albert Lasker Award for Clinical Medical Research, The Lasker Foundation. Retrieved 20 February 2008.
- ^ Houghton, M., Q.-L. Choo, and G. Kuo. NANBV Diagnostics and Vaccines. European Patent No. EP-0-3 18-216-A1. European Patent Office (filed 18 November 1988, published 31 May 1989).
- ^ Wilken, Judge. "United States Court of Appeals for the Federal Circuit". United States Court of Appeals for the Federal Circuit. Retrieved 11 January 2012.
- ^ Eurosurveillance editorial, team (2011 Jul 28). "World Hepatitis Day 2011". Euro surveillance : bulletin europeen sur les maladies transmissibles = European communicable disease bulletin. 16 (30). PMID 21813077.
{{cite journal}}: Check date values in:|date=(help) - ^ a b Wong, JB (2006). "Hepatitis C: cost of illness and considerations for the economic evaluation of antiviral therapies". PharmacoEconomics. 24 (7): 661–72. PMID 16802842.
- ^ a b El Khoury, A. C. (1 December 2011). "Economic burden of hepatitis C-associated diseases in the United States". Journal of Viral Hepatitis. doi:10.1111/j.1365-2893.2011.01563.x.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help)CS1 maint: date and year (link) - ^ "Hepatitis C Prevention, Support and Research ProgramHealth Canada". Public Health Agency of Canada. 2003. Retrieved 10 January 2012.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Zuckerman, edited by Howard Thomas, Stanley Lemon, Arie (2008). Viral Hepatitis (3rd ed. ed.). Oxford: John Wiley & Sons. p. 532. ISBN 9781405143882.
{{cite book}}:|edition=has extra text (help);|first=has generic name (help)CS1 maint: multiple names: authors list (link) - ^ Ahn, J (2011 Aug). "Hepatitis C therapy: other players in the game". Clinics in liver disease. 15 (3): 641–56. PMID 21867942.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Vermehren, J (2011 Feb). "New HCV therapies on the horizon". Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 17 (2): 122–34. PMID 21087349.
{{cite journal}}: Check date values in:|date=(help); Unknown parameter|coauthors=ignored (|author=suggested) (help)
