Maltase
| Alpha-glucosidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC no. | 3.2.1.20 | ||||||||
| CAS no. | 9001-42-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| |||||||||
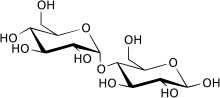
Maltase (EC 3.2.1.20, alpha-glucosidase, glucoinvertase, glucosidosucrase, maltase-glucoamylase, alpha-glucopyranosidase, glucosidoinvertase, alpha-D-glucosidase, alpha-glucoside hydrolase, alpha-1,4-glucosidase, alpha-D-glucoside glucohydrolase) is an enzyme located in on the brush border of the small intestine that breaks down the disaccharide maltose.[1][2][3][4][5][6] Maltase catalyzes the hydrolysis of maltose to the simple sugar glucose. This enzyme is found in plants, bacteria, and yeast. Acid maltase deficiency is categorized into three separate types based on the age of onset of symptoms in the affected individual.
In most cases, it is equivalent to alpha-glucosidase, but the term "maltase" emphasizes the disaccharide nature of the substrate from which glucose is cleaved, and "alpha-glucosidase" emphasizes the bond, whether the substrate is a disaccharide or polysaccharide.[citation needed]
Vampire bats are the only vertebrates known to not exhibit intestinal maltase activity.[7]
See also
References
- ^ "Maltase – Definition from the Merriam-Webster Online Dictionary". Retrieved 2009-04-06.
- ^ Bruni CB, Sica V, Auricchio F, Covelli I (September 1970). "Further kinetic and structural characterization of the lysosomal alpha-D-glucoside glucohydrolase from cattle liver". Biochimica et Biophysica Acta (BBA) - Enzymology. 212 (3): 470–7. doi:10.1016/0005-2744(70)90253-6. PMID 5466143.
- ^ Flanagan PR, Forstner GG (August 1978). "Purification of rat intestinal maltase/glucoamylase and its anomalous dissociation either by heat or by low pH". The Biochemical Journal. 173 (2): 553–63. doi:10.1042/bj1730553. PMC 1185809. PMID 29602.
- ^ Larner, J. (1960). "Other glucosidases". In Boyer, P.D.; Lardy, H.; Myrbäck, K. (eds.). The Enzymes. Vol. 4 (2nd ed.). New York: Academic Press. pp. 369–378.
- ^ Sivakami S, Radhakrishnan AN (December 1973). "Purification of rabbit intestinal glucoamylase by affinity chromatography on Sephadex G-200". Indian Journal of Biochemistry & Biophysics. 10 (4): 283–4. PMID 4792946.
- ^ Sørensen SH, Norén O, Sjöström H, Danielsen EM (September 1982). "Amphiphilic pig intestinal microvillus maltase/glucoamylase. Structure and specificity". European Journal of Biochemistry. 126 (3): 559–68. doi:10.1111/j.1432-1033.1982.tb06817.x. PMID 6814909.
- ^ Schondube JE, Herrera-M LG, Martínez del Rio C (2001). "Diet and the evolution of digestion and renal function in phyllostomid bats" (PDF). Zoology. 104 (1): 59–73. doi:10.1078/0944-2006-00007. PMID 16351819.
External links
- Maltases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Structure and evolution of the mammalian maltase-glucoamylase and sucrase-isomaltase
