From Wikipedia, the free encyclopedia
Chemical compound
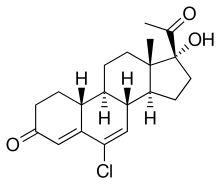
Amadinone Other names 6-Chloro-17α-hydroxy-19-norpregna-4,6-dione
(1S,2R,10R,11S,14R,15S)-14-acetyl-8-chloro-14-hydroxy-15-methyltetracyclo[8.7.0.02,7 .011,15 ]heptadeca-6,8-dien-5-one
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 20 H 25 Cl O 3 Molar mass −1 3D model (JSmol )
CC(=O)[C@]1(CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2C=C(C4=CC(=O)CC[C@H]34)Cl)C)O
InChI=1S/C20H25ClO3/c1-11(22)20(24)8-6-17-15-10-18(21)16-9-12(23)3-4-13(16)14(15)5-7-19(17,20)2/h9-10,13-15,17,24H,3-8H2,1-2H3/t13-,14-,15-,17+,19+,20+/m1/s1
Key:ANJGIFXUFSBZPX-WLCXVKOPSA-N
Amadinone (INN ), also known as 19-norchlormadinone , is a steroidal progestin of the 19-norprogesterone and 17α-hydroxyprogesterone groups that was synthesized and characterized in 1968 but was never marketed.[ 1] [ 2] antigonadotropic properties, and for this reason, is a functional antiandrogen .[ 3] [ 4] acetate ester , amadinone acetate , also exists, but similarly was never marketed.[ 1]
^ a b Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies ISBN 978-1-4757-2085-3 ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition ISBN 978-0-8155-1856-3 ^ Hughes A, Hasan SH, Oertel GW, Voss HE, Bahner F, Neumann G, Steinbeck H, Gräf KJ, Brotherton J, Horn HJ, Wagner RK (27 November 2013). Androgens II and Antiandrogens / Androgene II und Antiandrogene ISBN 978-3-642-80859-3 ^ Kent JR, Hill M, Huix FJ, Segre EJ (1972). "Seminal acid phosphatase content in the clinical bioassay of androgens and antiandrogens". Clinical Pharmacology and Therapeutics . 13 (2): 205–11. doi :10.1002/cpt1972132205 . PMID 5017374 . S2CID 40886901 .
PR Tooltip Progesterone receptor
Agonists
Testosterone derivatives: Progestins: 6,6-Difluoronorethisterone 6,6-Difluoronorethisterone acetate 17α-Allyl-19-nortestosterone Allylestrenol Altrenogest Chloroethynylnorgestrel Cingestol Danazol Desogestrel Dienogest Ethinylandrostenediol
Ethisterone Ethynerone Etonogestrel Etynodiol Etynodiol diacetate Gestodene Gestrinone Levonorgestrel Levonorgestrel esters (e.g., levonorgestrel butanoate )Lynestrenol Lynestrenol phenylpropionate Metynodiol Metynodiol diacetate Norelgestromin Norethisterone (norethindrone) Norethisterone esters (e.g., norethisterone acetate , norethisterone enanthate )Noretynodrel Norgesterone Norgestimate Norgestrel Norgestrienone Norvinisterone Oxendolone Quingestanol Quingestanol acetate Tibolone Tigestol Tosagestin ; Anabolic–androgenic steroids: 11β-Methyl-19-nortestosterone 11β-Methyl-19-nortestosterone dodecylcarbonate 19-Nor-5-androstenediol 19-Nor-5-androstenedione 19-Nordehydroepiandrosterone Bolandiol Bolandiol dipropionate Bolandione Dimethisterone Dienedione Dienolone Dimethandrolone Dimethandrolone buciclate Dimethandrolone dodecylcarbonate Dimethandrolone undecanoate Dimethyldienolone Dimethyltrienolone Ethyldienolone Ethylestrenol (ethylnandrol) Methyldienolone Metribolone (R-1881) Methoxydienone (methoxygonadiene) Mibolerone Nandrolone Nandrolone esters (e.g., nandrolone decanoate , nandrolone phenylpropionate )Norethandrolone Normethandrone (methylestrenolone, normethandrolone, normethisterone) RU-2309 Tetrahydrogestrinone Trenbolone (trienolone) Trenbolone esters (e.g., trenbolone acetate , trenbolone enanthate )Trendione Trestolone Trestolone acetate MixedSPRMs Tooltip Selective progesterone receptor modulators ) Antagonists
mPR Tooltip Membrane progesterone receptor PAQR Tooltip Progestin and adipoQ receptor )