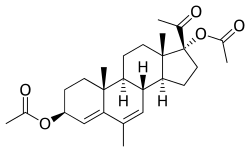
Acetomepregenol
 | |
| Clinical data | |
|---|---|
| Trade names | Diamol |
| Other names | ACM; Mepregenol diacetate; Diamol; Megestrol diacetate; Megestrol 3β,17α-diacetate; 3β,17α-Diacetoxy-6-methylpregna-4,6-dien-20-one; 6-Methylpregna-4,6-dien-3β,17α-diol-20-one diacetate |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C26H36O5 |
| Molar mass | 428.569 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Acetomepregenol (ACM), also known as mepregenol diacetate and sold under the brand name Diamol, is a progestin medication which is used in Russia for the treatment of gynecological conditions and as a method of birth control in combination with an estrogen.[1][2][3][4][5][6][7] It has also been studied in the treatment of threatened abortion.[3] It has been used in veterinary medicine as well.[8][9][10] It has been marketed since at least 1981.[8][9][10]
Pharmacology
Based on its chemical structure, namely the lack of a C3 ketone, it is probable that acetomepregenol is a prodrug of megestrol acetate (the 3-keto analogue).[11][12]
Chemistry
Acetomepregenol, also known as megestrol 3β,17α-diacetate, as well as 3β-dihydro-6-dehydro-6-methyl-17α-hydroxyprogesterone diacetate or as 3β,17α-diacetoxy-6-methylpregna-4,6-dien-20-one, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[3][4][7] It is very close to megestrol acetate (6-dehydro-6-methyl-17α-acetoxyprogesterone) in structure, except that there is a hydroxyl group with an acetate ester attached at the C3 position instead of a ketone.[3][4][7] A closely related medication is cymegesolate (also known as megestrol 3β-cypionate 17α-acetate), which, in contrast, has not been marketed.[13][14]
References
- ^ Korkhov VV (1985). "[Current trends in the development of oral contraception]". Farmakologiia I Toksikologiia (in Russian). 48 (4): 119–22. PMID 3899717.
- ^ Nikitina GV, Savchenko ON, Stepanov MG (1987). "[Hormonal properties of new 17 alpha-hydroxyprogesterone derivatives]". Problemy ĖNdokrinologii (in Russian). 33 (3): 60–3. PMID 3116530.
- ^ a b c d Sidel'nikova VM, Demidova EM, Borisova IuF, Dondukova TM, Absava GI, Korkhov VV (1990). "[The use of acetomepegrenol in the therapy of threatened abortion]". Akusherstvo I Ginekologii︠a︡ (in Russian) (9): 37–40. PMID 2278305.
- ^ a b c Grinenko, G. S.; Popova, E. V.; Korkhov, V. V.; Lesik, E. A.; Petrosyan, M. A.; Topil'skaya, N. I. (March 2000). "Synthesis and biological activity of 17α-acetoxy-3β-phenylpropionyloxy-6-methylpregna-4,6-dien-20-one". Pharmaceutical Chemistry Journal. 34 (3): 113–114. doi:10.1007/BF02524577. ISSN 1573-9031. S2CID 44235508.
Note that 3,17-diacetoxy-6-methylpregna-4,6-dien-20-one (1b), a structural analog of compound 1a, is certified in Russia under the trade name acetomepregnol and recommended for therapeutic purposes in gynecological practice and as a contraceptive preparation in combination with estrogens [4].
- ^ Mashkovskii, M. D. (December 2000). "Eightieth Anniversary of the Drug Chemistry Center/All-Russian Pharmaceutical Chemistry Scientific Research Institute". Pharmaceutical Chemistry Journal. 34 (12): 677–680. doi:10.1023/A:1010416205068. ISSN 1573-9031. S2CID 24703856.
- ^ Sergeev, P. V.; Rzheznikov, V. M.; Korkhov, V. V.; Grinenko, G. S.; Semeikin, A. V.; Mayatskaya, E. E.; Samoilikov, R. V.; Shimanovskii, N. L. (July 2005). "Investigation of the Gestagen Activity of 17α-acetoxy-3β-butanoyloxy-6-methylpregna-4,6-dien-20-one". Pharmaceutical Chemistry Journal. 39 (7): 358–360. doi:10.1007/s11094-005-0154-4. ISSN 1573-9031. S2CID 35450212.
Gestagens are widely used in medicine as drugs for the treatment of breast and uterine tumors, endometriosis, uterine bleeding, and premenstrual syndrome, as a means of hormonal therapy and maintenance of pregnancy, and as contraceptives [1, 2]. In clinics, drugs of this group are represented by acetomepregenol (AMP), medroxyprogesterone acetate (MPA), levonorgestrel, progesterone, didrogesterone, etc. [1].
- ^ a b c Zeinalov, O. A.; Yaderets, V. V.; Stytsenko, T. S.; Petrosyan, M. A.; Andryushina, V. A. (July 2012). "Synthesis and biological activity of synthetic 17α-hydroxyprogesterone derivatives". Pharmaceutical Chemistry Journal. 46 (4): 203–206. doi:10.1007/s11094-012-0761-9. ISSN 1573-9031. S2CID 7159432.
- ^ a b Bratanov, K., Bankov, N., Doichev, S., Pisheva, M., Klinskii, I. U., & Zhirkov, G. (1981). Action of diacetate mepregnol (diamol) on estrus induction in sheep in physiological anestrus. Reguliatsiia i intensifikatsiia protsessov razmnozheniia sel'skokhoziaistvennykh zhivotnykh: trudy Mezhdunarodnogo simpoziuma, sostoiavshegosia v Sofii, mai 1980 godina/[red. koll.: K. Bratanov (otvet. red.)... i dr.].
- ^ a b Zhirkov, G. F. (1981). Testing diamol on sheep on a fattening farm. Biulleten'nauchnykh rabot-Vsesoiuznyi nauchno-issledovatel'skii institut zhivotnovodstva.
- ^ a b Klinskii ID, Zhirkov GF (1982). "[Use of mepregenol diacetate (Diamol), a gestagen preparation, for estrus synchronization in caracul sheep during mating season]". Arch Exp Veterinarmed (in Russian). 36 (1): 159–62. PMID 7201304.
- ^ Ian S. Fraser (1998). Estrogens and Progestogens in Clinical Practice. Churchill Livingstone. p. 281. ISBN 978-0-443-04706-0.
Progestational activity depends on the presence of a 3-keto group in ring A of the steroid skeleton. Most of the progestogens used today do indeed carry such a group in their original molecules. However, the 3-keto group is initially missing in the case of desogestrel and norgestimate. They are prodrugs which undergo metabolic conversion to active 3-keto derivatives in the body.
- ^ McRobb L, Handelsman DJ, Kazlauskas R, Wilkinson S, McLeod MD, Heather AK (May 2008). "Structure-activity relationships of synthetic progestins in a yeast-based in vitro androgen bioassay". J. Steroid Biochem. Mol. Biol. 110 (1–2): 39–47. doi:10.1016/j.jsbmb.2007.10.008. PMID 18395441. S2CID 5612000.
Prodrugs (lack 3-keto): Ethylestrenol, Lynestrenol, Ethynodiol, Allylestrenol, Norgestimate
- ^ De-Wei Z (1982). "Research activities in the field of oral contraceptives in the People's Republic of China". Acta Obstet Gynecol Scand Suppl. 105: 51–60. doi:10.3109/00016348209155319. PMID 6952745. S2CID 44858028.
- ^ Yang, Yi-chien; Gu, Xi-gen; Li, Shu-xiang (1982). Proteins and Steroids in Early Pregnancy. pp. 335–342. doi:10.1007/978-3-642-67890-5_22. ISBN 978-3-642-67892-9.
