Icometasone
Appearance
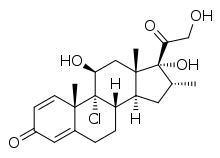 | |
| Clinical data | |
|---|---|
| Other names | Icomethasone; 9α-Chloro-11β,17α,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione |
| Drug class | Corticosteroid; Glucocorticoid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C22H29ClO5 |
| Molar mass | 408.92 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Icometasone is a synthetic glucocorticoid corticosteroid which was never marketed.[1][2][3]
References
- ^ Duchêne P, Giudicelli MD, Neau B, Gronfier A, Firmin Y, Villax P, Saivin S, Houin G (1998). "Pharmacokinetics, protein binding and metabolic profile of 3H-icometasone enbutate following intravenous, oral and intratracheal administrations to Sprague-Dawley rats". Arzneimittelforschung. 48 (4): 371–8. PMID 9608879.
- ^ Martin Negwer; Hans-Georg Scharnow (2001). Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. p. 3730. ISBN 978-3-527-30247-5.
- ^ The use of stems in the selection of international Nonproprietary Names (INN) for pharmaceutical substances (PDF) (Report). World Health Organization. 2013. WHO/EMP/RHT/TSN/2013.1. Archived from the original (PDF) on 8 March 2022.
