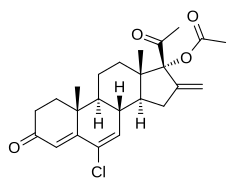
Chlormethenmadinone acetate
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Biogest, Sterolibrin, Antigest B, Agelin |
| Other names | SCH-12600; 6-Chloromethylenedehydroacetoxyprogesterone; 17α-Acetoxy-6-chloro-16-methylene-6-dehydroprogesterone; 16-Methylenechlormadinone acetate; 17α-Acetoxy-6-chloro-16-methylenepregna-4,6-diene-3,20-dione |
| Drug class | Progestogen; Progestin; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C24H29ClO4 |
| Molar mass | 416.94 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Chlormethenmadinone acetate (CMMA), also known as chlorsuperlutin, is a progestin medication which was developed in Czechoslovakia in the 1960s.[1] It has been used in combination with mestranol in birth control pills under the brand names Biogest, Sterolibrin, and Antigest B,[2][3] and in veterinary medicine under the brand name Agelin.[4] Analogues of CMMA include bromethenmadinone acetate (bromsuperlutin), which was assessed but was never marketed,[3][5] and melengestrol acetate (methylsuperlutin), which is used in veterinary medicine.[6]
See also
- List of progestogen esters § Esters of 17α-hydroxyprogesterone derivatives
- 16-Methylene-17α-hydroxyprogesterone acetate
References
- ^ Sterba, R. (1968). New biological application of contraceptive steroids. Endocrinologia Experimentalis, 2(2), 101-110. https://www.popline.org/node/469522 Archived 2018-09-16 at the Wayback Machine
- ^ Melich H (July 1972). "[Biogest]". Cas. Lek. Cesk. (in Czech). 111 (30): 694–5. PMID 5079918. Archived from the original on 2018-09-16. Retrieved 2018-09-16.
- ^ a b Stĕrba R (March 1970). "[Towards a more physiological hormonal contraception]". Zentralbl Gynakol (in German). 92 (10): 303–12. PMID 4096927. Archived from the original on 2018-09-16. Retrieved 2018-09-16.
- ^ Bekeová E, Krajnicáková M, Hendrichovský V, Maracek I (November 1995). "[Thyroid and ovarian hormones in ewes treated with gestagens and PMSG in the spring season]". Vet Med (Praha) (in Slovak). 40 (11): 345–52. PMID 8659087.
- ^ Štěrba, R. (1971). "On the Way to a More Physiological Hormonal Contraception". Current Problems in Fertility. pp. 154–158. doi:10.1007/978-1-4615-8651-7_28. ISBN 978-1-4615-8653-1. Archived from the original on 2018-09-16. Retrieved 2018-09-16.
- ^ R. G. Denkewalter; M. Tishler; G. Ehrhart; J. H. Biel, B. K. B. Lum, J. Büchi, C. A. Winter, K. Münzel, W. Kunz, E. J. Ariëns, F. Labhardt (8 March 2013). Fortschritte der Arzneimittelforschung / Progress in Drug Research / Progrès des recherches pharmaceutiques. Birkhäuser. pp. 407–. ISBN 978-3-0348-7059-7.
{{cite book}}: CS1 maint: multiple names: authors list (link)
