ZMapp
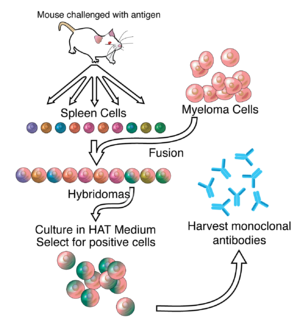
ZMapp is an experimental biopharmaceutical drug comprising three chimeric monoclonal antibodies under development as a treatment for Ebola virus disease.[1] Two of the three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and the third at the U.S. Army Medical Research Institute of Infectious Diseases; the cocktail was optimized by Gary Kobinger, a research scientist at the NML[2] and underwent further development under license by Mapp Biopharmaceutical. ZMapp was first used on humans during the Western African Ebola virus epidemic, having only been previously tested on animals and not yet subjected to a randomized controlled trial.[3] The National Institutes of Health (NIH) ran a clinical trial starting in January 2015 with subjects from Sierra Leone, Guinea, and Liberia aiming to enroll 200 people, but the epidemic waned and the trial closed early, leaving it too statistically underpowered to give a meaningful result about whether ZMapp worked.[4]
In 2016, a clinical study comparing ZMapp to the current standard of care for Ebola was inconclusive.[5]
Chemistry
The drug is composed of three monoclonal antibodies (mAbs), initially harvested from mice exposed to Ebola virus proteins, that have been chimerized with human constant regions.[6] The components are chimeric monoclonal antibody c13C6 from a previously existing antibody cocktail called "MB-003" and two chimeric mAbs from a different antibody cocktail called ZMab, c2G4, and c4G7.[7] ZMapp is manufactured in the tobacco plant Nicotiana benthamiana in the bioproduction process known as "pharming" by Kentucky BioProcessing, a subsidiary of Reynolds American.[1][8][9]
Mechanism of action
Like intravenous immunoglobulin therapy, ZMapp contains a mixture of neutralizing antibodies that confer passive immunity to an individual, enhancing the normal immune response, and is designed to be administered after exposure to the Ebola virus.[10] Such antibodies have been used in the treatment and prevention of various infectious diseases and are intended to attack the virus by interfering with its surface and neutralizing it to prevent further damage.[10][11]

History
Two of the drug's three components were originally developed at the Public Health Agency of Canada's National Microbiology Laboratory (NML), and a third at the U.S. Army Medical Research Institute of Infectious Diseases;[2] the cocktail was optimized by Gary Kobinger, then branch chief of the NML, and is undergoing further development by Leaf Biopharmaceutical (LeafBio, Inc.), a San Diego-based arm of Mapp Biopharmaceutical.[12] LeafBio created ZMapp in collaboration with its parent and Defyrus Inc., each of which had licensed its own cocktail of antibodies, called MB-003 and ZMab.[13][citation needed]
MB-003
MB-003 is a cocktail of three humanized or human–mouse chimeric mAbs: c13C6, h13F6, and c6D8.[7] A study published in September 2012 found that rhesus macaques infected with Ebola virus (EBOV) survived when receiving MB-003 (mixture of 3 chimeric monoclonal antibodies) one hour after infection. When treated 24 or 48 hours after infection, four of six animals survived and had little to no viremia and few, if any, clinical symptoms.[14]
MB-003 was created by scientists at the U.S. Army Medical Research Institute of Infectious Diseases, Gene Olinger, and Jamie Pettitt in collaboration with Mapp Biopharmaceutical with years of funding from US government agencies including the National Institute of Allergy and Infectious Disease, Biomedical Advanced Research and Development Authority, and the Defense Threat Reduction Agency.[1][2][15]
ZMAb
ZMAb is a mixture of three mouse mAbs: m1H3, m2G4, and m4G7.[7] A study published in November 2013 found that EBOV-infected macaque monkeys survived after being given a therapy with a combination of three EBOV surface glycoprotein (EBOV-GP)-specific monoclonal antibodies (ZMAb) within 24 hours of infection. The authors concluded that post-exposure treatment resulted in a robust immune response, with good protection for up to 10 weeks and some protection at 13 weeks.[16] ZMab was created by the NML and licensed to Defyrus, a Toronto-based biodefense company, with further funding by the Public Health Agency of Canada.[2]
ZMapp
A 2014 paper described how Mapp and its collaborators, including investigators at Public Health Agency of Canada, Kentucky BioProcessing, and the National Institute of Allergy and Infectious Diseases, first chimerized the three antibodies comprising ZMAb, then tested combinations of MB-003 and the chimeric ZMAb antibodies in guinea pigs and then primates to determine the best combination, which turned out to be c13C6 from MB-003 and two chimeric mAbs from ZMAb, c2G4 and c4G7. This is ZMapp.[7]
In an experiment also published in the 2014 paper, 21 rhesus macaque primates were infected with the Kikwit Congolese variant of EBOV. Three primates in the control arm were given a non-functional antibody, and the 18 in the treatment arm were divided into three groups of six. All primates in the treatment arm received three doses of ZMapp, spaced 3 days apart. The first treatment group received its first dose on 3rd day after being infected; the second group on the 4th day after being infected, and the third group, on the 5th day after being infected. All three primates in the control group died; all 18 primates in the treatment arm survived.[7] Mapp then went on to show that ZMapp inhibits replication of a Guinean strain of EBOV in cell cultures.[17]
Mapp remains involved in the production of the drug through its contracts with Kentucky BioProcessing, a subsidiary of Reynolds American.[1] To produce the drug, genes coding for the chimeric mAbs were inserted into viral vectors, and tobacco plants are infected with the viral vector encoding for the antibodies, using Agrobacterium cultures.[18][19][20] Subsequently, antibodies are extracted and purified from the plants. Once the genes encoding the chimeric mAbs are in hand, the entire tobacco production cycle is believed to take a few months.[3] The development of these production methods was funded by the U.S. Defense Advanced Research Projects Agency as part of its bio-defense efforts following the 9/11 terrorist attacks.[21][22]
Use during the 2014–16 Ebola outbreak in West Africa
ZMapp was first used during the 2014 West Africa Ebola Virus outbreak, having not previously undergone any human clinical trials to determine its efficacy or potential risks.[3] By October 2014, the United States Food and Drug Administration had approved the use of several experimental drugs, including ZMapp, to be used on patients infected with Ebola virus. The use of such drugs during the epidemic was also deemed ethical by the World Health Organization.[23] In 2014, a limited supply of ZMapp was used to treat 7 individuals infected with the Ebola virus; of these 2 died.[24][25] The outcome is not considered to be statistically significant. Mapp announced in August 2014, that supplies of ZMapp had been exhausted.[26]
Controversy
The lack of drugs and unavailability of experimental treatment in the most affected regions of the West African Ebola virus outbreak spurred some controversy.[3] The fact that the drug was first given to Americans and a European and not to Africans, according to the Los Angeles Times, "provoked outrage, feeding into African perceptions of Western insensitivity and arrogance, with a deep sense of mistrust and betrayal still lingering over the exploitation and abuses of the colonial era".[27] Salim S. Abdool Karim, the director of an AIDS research center in South Africa, placed the issue in the context of the history of exploitation and abuses. Responding to a question on how people might have reacted if ZMapp and other drugs had first been used on Africans, he said "It would have been the front-page screaming headline: 'Africans used as guinea pigs for American drug company's medicine'".[3]
In early August, the World Health Organization called for convening a panel of medical authorities "to consider whether experimental drugs should be more widely released." In a statement, Peter Piot (co-discoverer of the Ebola virus); Jeremy Farrar, the director of the Wellcome Trust; and David Heymann of the Chatham House Center on Global Health Security, called for the release of experimental drugs for affected African nations.[27]
At an August 6, 2014 press conference, Barack Obama, the President of the United States, was questioned regarding whether the cocktail should be fast-tracked for approval or be made available to sick patients outside of the United States. He responded, "I think we've got to let the science guide us. I don't think all the information's in on whether this drug is helpful."[28]
Clinical trial
The National Institutes of Health announced on 27 February 2015 the commencement of a randomized controlled trial of ZMapp to be conducted in Liberia and the United States.[29] From March 2015 through November 2015, 72 individuals infected with the Ebola virus were enrolled in the trial; investigators stopped enrolling new subjects in January 2016, having failed to reach its enrollment goal of 200 due to the waning of the Ebola outbreak. As a result, although a 40% lower risk of death was calculated for those who received ZMapp, the difference was not statistically significant and ultimately it could not be determined whether the use of ZMapp was superior to the optimized standard of care alone. However, ZMapp was found to be safe and well tolerated.[4][30][31]
Use during the 2018-19 Kivu Ebola outbreak in the Democratic Republic of the Congo
The ZMapp cocktail was assessed by the World Health Organization for emergency use under the MEURI ethical protocol. The panel agreed that "the benefits of ZMapp outweigh its risks" while noting that it presented logistical challenges, particularly that of requiring a cold chain for distribution and storage.[32] Four alternative therapies (remdesivir, the Regeneron product atoltivimab/maftivimab/odesivimab, favipiravir, and ansuvimab) were also considered for use, but they were at earlier stages of development.[32] In August 2019, the Democratic Republic of the Congo's national health authorities, the World Health Organization, and the National Institutes of Health announced that they would stop using ZMapp, along with all other Ebola treatments except atoltivimab/maftivimab/odesivimab and ansuvimab, in their ongoing clinical trials, citing the higher mortality rates of patients not treated with atoltivimab/maftivimab/odesivimab and ansuvimab.[33][34]
In October 2020, the U.S. Food and Drug Administration (FDA) approved atoltivimab/maftivimab/odesivimab with an indication for the treatment of infection caused by Zaire ebolavirus.[35]
See also
References
- ^ a b c d e Kroll, David (5 August 2014). "Ebola 'Secret Serum': Small Biopharma, The Army, And Big Tobacco". Forbes.
- ^ a b c d Branswell, Helen (September 21, 2014). "How a Winnipeg lab became an Ebola research powerhouse". CBC News. The Canadian Press. Retrieved 23 November 2015.
- ^ a b c d e Pollack, Andrew (8 August 2014). "In Ebola outbreak, who should get experimental drug?". The New York Times.
- ^ a b "Press release: Study Finds Ebola Treatment ZMapp Holds Promise, Although Results Not Definitive | NIH: National Institute of Allergy and Infectious Diseases". National Institute of Allergy and Infectious Diseases. October 12, 2016.
- ^ PREVAIL II (October 13, 2016). "A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection". NEJM. 375 (15): 1448–1456. doi:10.1056/NEJMoa1604330. PMC 5086427. PMID 27732819.
- ^ Pollack, Andrew (29 August 2014). "Experimental Drug Would Help Fight Ebola if Supply Increases, Study Finds". The New York Times. Retrieved 1 September 2014.
- ^ a b c d e Qiu X, Wong G, Audet J, et al. (October 2014). "Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp". Nature. 514 (7520): 47–53. Bibcode:2014Natur.514...47Q. doi:10.1038/nature13777. PMC 4214273. PMID 25171469.
- ^ Parshley, Lois (8 August 2014). "ZMapp: The Experimental Ebola Treatment Explained". Popular Science.
- ^ Daniel, Fran (12 August 2014). "Ebola drug provided for two Americans by Reynolds American subsidiary". Winston-Salem Journal.
- ^ a b "Ebola 'cocktail' developed at Canadian and U.S. labs". CBC News. 2014-08-05.
- ^ Keller MA, Stiehm ER (October 2000). "Passive immunity in prevention and treatment of infectious diseases". Clin. Microbiol. Rev. 13 (4): 602–14. doi:10.1128/CMR.13.4.602-614.2000. PMC 88952. PMID 11023960.
- ^ "Monoclonal antibody-based filovirus therapeutic licensed to Leaf Biopharmaceutical" (PDF). 15 July 2014.
- ^ Davidson E, Bryan C, Fong RH, et al. (November 2015). "Mechanism of Binding to Ebola Virus Glycoprotein by the ZMapp, ZMAb, and MB-003 Cocktail Antibodies". J. Virol. 89 (21): 10982–92. doi:10.1128/JVI.01490-15. PMC 4621129. PMID 26311869.
- ^ Olinger GG, Pettitt J, Kim D, et al. (October 2012). "Delayed treatment of Ebola virus infection with plant-derived monoclonal antibodies provides protection in rhesus macaques". Proc. Natl. Acad. Sci. U.S.A. 109 (44): 18030–5. Bibcode:2012PNAS..10918030O. doi:10.1073/pnas.1213709109. PMC 3497800. PMID 23071322.
- ^ "Questions and answers on experimental treatments and vaccines for Ebola". Centers for Disease Control and Prevention. 29 August 2014.
- ^ Qiu X, Audet J, Wong G, et al. (November 2013). "Sustained protection against Ebola virus infection following treatment of infected nonhuman primates with ZMAb". Sci Rep. 3: 3365. Bibcode:2013NatSR...3E3365Q. doi:10.1038/srep03365. PMC 3842534. PMID 24284388.
- ^ Geisbert TW (August 2014). "Medical research: Ebola therapy protects severely ill monkeys". Nature. 514 (7520): 41–43. Bibcode:2014Natur.514...41G. doi:10.1038/nature13746. PMC 4469351. PMID 25171470.
- ^ "Magnifection – mass-producing drugs in record time". National Geographic. 2008-08-16. Retrieved 11 September 2014.
- ^ Whaley KJ, et al. (2014). "Emerging antibody-based products". Plant Viral Vectors. Current Topics in Microbiology and Immunology. Vol. 375. pp. 107–26. doi:10.1007/82_2012_240. ISBN 978-3-642-40828-1. PMID 22772797.
{{cite book}}:|journal=ignored (help) - ^ Giritch A, Marillonnet S, Engler C, et al. (October 2006). "Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors". Proc. Natl. Acad. Sci. U.S.A. 103 (40): 14701–6. doi:10.1073/pnas.0606631103. PMC 1566189. PMID 16973752.
- ^ Till, Brian (9 September 2014). "DARPA may have a way to stop Ebola in its tracks". New Republic.
- ^ Shannon Pettypiece for Bloomberg News. 23, October 2014. Cheney in Post 9/11 Era May Be to Thank on Ebola Vaccine
- ^ Lai, K. K. Rebecca (2014-10-23). "Treating Ebola: The Hunt for a Drug". The New York Times. ISSN 0362-4331. Retrieved 2017-11-15.
- ^ "Spain imports US experimental Ebola drug to treat priest evacuated from Liberia with disease". First Word Pharma. 11 August 2014.
- ^ McCarthy, M. (2014). "US signs contract with ZMapp maker to accelerate development of the Ebola drug". BMJ. 349: g5488. doi:10.1136/bmj.g5488. PMID 25189475. S2CID 28961384.
{{cite journal}}: Unknown parameter|agency=ignored (help) - ^ "How Will We Know If The Ebola Drugs Worked?". Forbes. Retrieved 10 September 2014.
- ^ a b Dixon, Robyn (6 August 2014). "Three leading Ebola experts call for release of experimental drug". Los Angeles Times. Johannesburg.
- ^ "Obama: 'Premature' to say U.S. should green-light new Ebola drug". NBC News. 6 August 2014.
- ^ "Liberia-U.S. clinical research partnership opens trial to test Ebola treatments". National Institutes of Health (NIH). Retrieved 27 February 2015.
- ^ Bixler, Sandra L.; Duplantier, Allen J.; Bavari, Sina (2017). "Discovering Drugs for the Treatment of Ebola Virus". Current Treatment Options in Infectious Diseases. 9 (3): 299–317. doi:10.1007/s40506-017-0130-z. ISSN 1534-6250. PMC 5570806. PMID 28890666.
- ^ Davey RT, Dodd L, Proschan MA, et al. (October 2016). "A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection". N. Engl. J. Med. 375 (15): 1448–1456. doi:10.1056/NEJMoa1604330. PMC 5086427. PMID 27732819.
- ^ a b "Notes for the record: Consultation on Monitored Emergency Use of Unregistered and Investigational Interventions for Ebola Virus Disease (EVD)" (PDF). World Health Organization. 17 May 2018. Retrieved 25 May 2018.
- ^ McNeil, Donald G. Jr. (12 August 2019). "A Cure for Ebola? Two New Treatments Prove Highly Effective in Congo". The New York Times. Retrieved 13 August 2019.
- ^ Molteni, Megan (12 August 2019). "Ebola is Now Curable. Here's How The New Treatments Work". Wired. Retrieved 13 August 2019.
- ^ "FDA Approves First Treatment for Ebola Virus". U.S. Food and Drug Administration (FDA) (Press release). 14 October 2020. Retrieved 14 October 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
Further reading
- Madelain V, Nguyen TH, Olivo A, et al. (August 2016). "Ebola Virus Infection: Review of the Pharmacokinetic and Pharmacodynamic Properties of Drugs Considered for Testing in Human Efficacy Trials". Clin Pharmacokinet. 55 (8): 907–23. doi:10.1007/s40262-015-0364-1. PMC 5680399. PMID 26798032.

