Thulium(III) oxide
| |
| Names | |
|---|---|
| Other names
Thulium oxide, thulium sesquioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ECHA InfoCard | 100.031.670 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Tm2O3 | |
| Molar mass | 385.866 g/mol |
| Appearance | greenish-white cubic crystals |
| Density | 8.6 g/cm3 |
| Melting point | 2,341 °C (4,246 °F; 2,614 K) |
| Boiling point | 3,945 °C (7,133 °F; 4,218 K) |
| slightly soluble in acids | |
| +51,444·10−6 cm3/mol | |
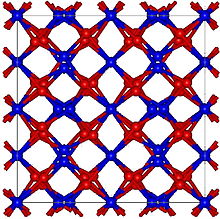
| Structure | |
| Cubic, cI80 | |
| Ia-3, No. 206 | |
| Related compounds | |
Other anions
|
Thulium(III) chloride |
Other cations
|
Erbium(III) oxide Ytterbium(III) oxide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Thulium(III) oxide is a pale green solid compound, with the formula Tm2O3. It was first isolated in 1879 from an impure sample of erbia by Per Teodor Cleve, who named it thulia. It can be prepared in the laboratory by burning thulium metal in air, or by decomposition of their oxoacid salts, such as thulium nitrate.[1]
References
- ^ Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 25: The f-block metals: lanthanoids and actinoids". Inorganic Chemistry, 3rd Edition. Pearson. p. 864. ISBN 978-0-13-175553-6.
