Α-Amanitin
| |

| |
| Names | |
|---|---|
| Other names
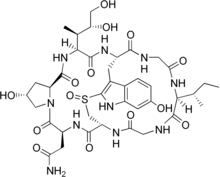
(cyclic(L)-asparaginyl-4-hydroxy-L-proly-(R)-4,5-dihydroxy-L-isoleucyl-6-hydroxy-2-mercapto-L-tryptophylglycyl-L-isoleucylglycyl-L-cysteinyl) cyclic (4 → 8)-sulfide(R)-S-oxide.
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.041.287 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C39H54N10O14S | |
| Molar mass | 918.97 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
alpha-Amanitin or α-amanitin is a cyclic peptide of eight amino acids. It is possibly the most deadly of all the amatoxins, toxins found in several species of the Amanita genus of mushrooms, one being the death cap (Amanita phalloides) as well as the destroying angel, a complex of similar species, principally A. virosa and A. bisporigera. It is also found in the mushrooms Galerina marginata and Conocybe filaris. The oral LD50 of amanitin is approximately 0.1 mg/kg for rats.
The structure of the polypeptide is atypical of most polypeptides, due to the branching of the amino acid chain. A cross bridge between 6-hydroxy-tryptophan and cysteine allows the formation of a second "inner loop," as seen in the diagram at right. The "outer loop" is formed by the normal peptide bond of the carboxyl terminus to the amino terminus of the peptide chain.
Unlike most cyclic peptides, amatoxins (and phallotoxins) are synthesized on ribosomes. Heather Hallen and Jonathan Walton of Michigan State University, first isolated the genes encoding the proproteins for α-amanitin and phallacidin.[1]
Scientific use
α-Amanitin is an inhibitor of RNA polymerase II and III.[2] This mechanism makes it a deadly toxin.
α-Amanitin can also be used to determine which types of RNA polymerase are present. This is done by testing the sensitivity of the polymerase in the presence of α-amanitin. RNA polymerase I is insensitive, RNA polymerase II is highly sensitive (inhibited at 1μg/ml), RNA polymerase III is moderately sensitive (inhibited at 10μg/ml), and RNA polymerase IV is slightly sensitive (inhibited at 50μg/ml).[citation needed]
Symptoms of poisoning
α-Amanitin has an unusually strong and specific attraction to the enzyme RNA polymerase II. Upon ingestion and uptake by liver cells, it binds to the RNA polymerase II enzyme, effectively causing cytolysis of hepatocytes (liver cells).[3] Few effects are reported within 10 hours; it is not unusual for significant effects to take as long as 24 hours after ingestion to appear, with this delay in symptoms making α-amanitin poisoning even more difficult to diagnose and all the more dangerous. By then, it is far past the time in which stomach pumping would yield an efficient result. Diarrhea and cramps are the first symptoms, but those pass, giving a false sign of remission. Typically, on the 4th to 5th day, the toxin starts to have severe effects on the liver and kidneys, leading to total system failure in both. Death usually takes place around a week from ingestion.[4]
Around 15% of those poisoned will die within 10 days, progressing through a comatose stage to kidney failure, liver failure, hepatic coma, respiratory failure and death. Those who recover are at risk of permanent liver damage.[5] Diagnosis is difficult, and is established by observation of the clinical symptoms as well as the presence of α-amanitin in the urine. Urine screening is generally most useful within 48 hours of ingestion. Treatment is mainly supportive (gastric lavage, activated carbon, fluid resuscitation) but includes various drugs to counter the amatoxins, including intravenous penicillin and cephalosporin derivatives, and, in cases of greater ingestion, can extend to an orthotopic liver transplant. The most reliable method to treat amanitin poisoning is through having the stomach pumped immediately after ingestion; however, the onset of symptoms is generally too late for this to be an option. Chemically modified silibinin, silibinin dihydrogen disuccinate disodium (trade name Legalon SIL) a solution for IV administration, is used in treatment of severe intoxications with hepatotoxic substances such as paracetamol and to treat Amanita phalloides poisoning.[6]
Mode of inhibitory action

From the crystal structure solved by Dr. Bushnell et al.,[7] α-Amanitin interacts with the bridge helix in RNA polymerase II (pol II). This interaction interferes with the translocation of RNA and DNA needed to empty the site for the next round of RNA synthesis. The addition of α-amanitin can reduce the rate of pol II translocating on DNA from several thousand to a few nucleotides per minute,[8][9] but has little effect on the affinity of pol II for nucleoside triphosphate,[10] and a phosphodiester bond can still be formed.[11][12] The bridge helix has evolved to be flexible and its movement is required for translocation of the polymerase along the DNA backbone. Binding of α-amanitin puts a constraint on its mobility, hence slowing down the translocation of the polymerase and the rate of synthesis of the RNA molecule.
Use in Medicine
Heidelberg Pharma, GmbH, based in Ladenburg, Germany, a pharmaceutical company providing pre-clinical drug discovery and development services, has developed a new antibody-drug conjugate or ADC technology based on α-amanitin. Amanitin-based ADCs have shown outstanding activity in therapy-resistant tumor cells, e.g. cells expressing multi-drug resistant transporters, tumor-initiating cells and non-dividing cells at picomolar concentrations.[13]
The unique mode of action or MOA of α-amanitin seems to make the amanitin-based antibody-drug conjugates a suitable toxic payload. The tolerability and therapeutic window of amanitin-based ADCs has been determined in a variety of rodent and non-human primate models. Furthermore, amanitin has a water-soluble structure, resulting in antibody-drug conjugates with low tendency for aggregation, even using higher drug to antibody ratios or DAR.[14][15]
In preclinical mouse models of prostate cancer, α-(alpha)-amanitin conjugated to an antibody directed against prostate-specific membrane antigen (PSMA; FOLH1; GCPII) showed high antitumoral activity and caused complete remission at single i.v. doses of 150 μg/kg of toxin, with no more than marginal weight loss in treated animals. Also, amanitin-based antibody-drug conjugates using an anti-Her2 antibody such as trastuzumab showed high antitumor activity in a series of models of preclinical oncology designed to establish efficacy of the trial drug in the treatment of HER2+ breast cancer. Alpha-amanitin is highly active in drug-resistant cells, independent of the status of expression of multi-drug resistant transporters because of its hydrophilic structure. Inhibition of RNA polymerase II amanitin-binding not only leads to apoptosis of dividing cells, but also of slowly growing cells – which is often observed in prostate cancer.[16][17]
See also
References
- ^ H. Hallen, H. Luo, J.S. Scott-Craig, and J.D. Walton (2007). "A gene family encoding the major toxins of lethal Amanita mushrooms". Proceedings of the National Academy of Sciences of the United States of America. 104 (48): 19097–19101. doi:10.1073/pnas.0707340104. PMC 2141914. PMID 18025465.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ B. Meinecke and S. Meinecke-Tillmann (1993). "Effects of -amanitin on nuclear maturation of porcine oocytes in vitro". Journal of Reproduction and Fertility. 98 (1): 195–201. doi:10.1530/jrf.0.0980195. PMID 8345464.
- ^ D. Michelot and R. Labia (1988). "alpha-Amanitin: a possible suicide substrate-like toxin involving the sulphoxide moiety of the bridged cyclopeptide". Drug Metabol Drug Interact. 6 (3–4): 265–274. doi:10.1515/dmdi.1988.6.3-4.265. PMID 3078291.
- ^ A. Mas (2005). "Mushrooms". Journal of Hepatology. 42 (2): 166–169. doi:10.1016/j.jhep.2004.12.003. PMID 15664239.
- ^ Benjamin DR. "Amatoxin syndrome": 198–214.
{{cite journal}}: Cite journal requires|journal=(help) in: Mushrooms: poisons and panaceas — a handbook for naturalists, mycologists and physicians. New York: WH Freeman and Company. 1995. - ^ Mitchell, T (2009). "Intravenous Milk thistle (silibinin-legalon) for hepatic failure induced by Amanita mushroom poisoning". (Clinical study).
- ^ a b Bushnell, D. A.; Cramer, P; Kornberg, RD (Feb 2002). "Structural basis of transcription: alpha-amanitin-RNA polymerase II cocrystal at 2.8 A resolution". Proc Natl Acad Sci USA. 99 (3): 1218–1222. doi:10.1073/pnas.251664698. PMC 122170. PMID 11805306.
- ^ Chafin, D. R. , Guo, H. & Price, D. H. (1995). "Action of alpha-Amanitin during Pyrophosphorolysis and Elongation by RNA Polymerase II". J. Biol. Chem. 270 (32): 19114–19119. doi:10.1074/jbc.270.32.19114. PMID 7642577.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Rudd, M. D. & Luse, D. S. (1996). "Amanitin Greatly Reduces the Rate of Transcription by RNA Polymerase II Ternary Complexes but Fails to Inhibit Some Transcript Cleavage Modes". J. Biol. Chem. 271 (35): 21549–21558. doi:10.1074/jbc.271.35.21549. PMID 8702941.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Cochet-Meilhac, M. & Chambon, P. (1974). "Animal DNA-dependent RNA polymerases. 11. Mechanism of the inhibition of RNA polymerases B by amatoxins". Biochim. Biophys. Acta. 353 (2): 160–184. doi:10.1016/0005-2787(74)90182-8. PMID 4601749.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vaisius, A. C. & Wieland, T. (1982). "Formation of a single phosphodiester bond by RNA polymerase B from calf thymus is not inhibited by .alpha.-amanitin". Biochemistry. 21 (13): 3097–3101. doi:10.1021/bi00256a010. PMID 7104312.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gu, W. , Powell, W. , Mote, J., Jr. & Reines, D. (1993). "Nascent RNA cleavage by arrested RNA polymerase II does not require upstream translocation of the elongation complex on DNA". J. Biol. Chem. 268 (34): 25604–25616. PMID 7503982.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Alpha-amanitin. ADC Review / Journal of Antibody-drug Conjugates
- ^ Moldenhauer G, Salnikov AV, Lüttgau S, Herr I, Anderl J, Faulstich H. Therapeutic potential of amanitin-conjugated anti-epithelial cell adhesion molecule monoclonal antibody against pancreatic carcinoma. J Natl Cancer Inst. 2012; 104(8):622-34
- ^ Hechler T, Kulke M, Müller C, Pahl A, Anderl J. Amanitin-based antibody-drug conjugates targeting the prostate-specific membrane antigen PSMA. Poster #664, AACR Annual Meeting 2014
- ^ Hechler T, Kulke M, Müller C, Pahl A, Anderl J. Amanitin-based ADCs with an improved therapeutic index. Poster #633, AACR Annual Meeting 2015
- ^ Anderl J, Faulstich H, Hechler T, Kulke M. Antibody–Drug Conjugate Payloads. Methods Mol Biol. 2013; 1045:51-70

