Cadmium
 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cadmium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈkædmiəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery bluish-gray metallic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Cd) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cadmium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 48 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 12 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Kr] 4d10 5s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 594.22 K (321.07 °C, 609.93 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 1040 K (767 °C, 1413 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 8.649 g/cm3[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 7.996 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 6.21 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 99.87 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 26.020 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | common: +2 −2,? +1? | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.69 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 151 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 144±9 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 158 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal close-packed (hcp) (hP2) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constants | a = 297.89 pm c = 561.66 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 30.95×10−6/K (at 20 °C)[3][a] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 96.6 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 72.7 nΩ⋅m (at 22 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −19.8×10−6 cm3/mol[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 50 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 19 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 42 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 2310 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.30 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Brinell hardness | 203–220 MPa | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-43-9 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery and first isolation | Karl Samuel Leberecht Hermann and Friedrich Stromeyer (1817) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Named by | Friedrich Stromeyer (1817) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of cadmium | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Cadmium is a chemical element with symbol Cd and atomic number 48. This soft, bluish-white metal is chemically similar to the two other stable metals in group 12, zinc and mercury. Like zinc, it demonstrates oxidation state +2 in most of its compounds, and like mercury, it has a lower melting point than other transition metals. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states. The average concentration of cadmium in Earth's crust is between 0.1 and 0.5 parts per million (ppm). It was discovered in 1817 simultaneously by Stromeyer and Hermann, both in Germany, as an impurity in zinc carbonate.
Cadmium occurs as a minor component in most zinc ores and is a byproduct of zinc production. Cadmium was used for a long time as a corrosion-resistant plating on steel, and cadmium compounds are used as red, orange and yellow pigments, to colour glass, and to stabilize plastic. Cadmium use is generally decreasing because it is toxic (it is specifically listed in the European Restriction of Hazardous Substances[7]) and nickel-cadmium batteries have been replaced with nickel-metal hydride and lithium-ion batteries. One of its few new uses is cadmium telluride solar panels.
Although cadmium has no known biological function in higher organisms, a cadmium-dependent carbonic anhydrase has been found in marine diatoms.
Characteristics
Physical properties
Cadmium is a soft, malleable, ductile, bluish-white divalent metal. It is similar in many respects to zinc but forms complex compounds.[8] Unlike most other metals, cadmium is resistant to corrosion and is used as a protective plate on other metals. As a bulk metal, cadmium is insoluble in water and is not flammable; however, in its powdered form it may burn and release toxic fumes.[9]
Chemical properties
Although cadmium usually has an oxidation state of +2, it also exists in the +1 state. Cadmium and its congeners are not always considered transition metals, in that they do not have partly filled d or f electron shells in the elemental or common oxidation states.[10] Cadmium burns in air to form brown amorphous cadmium oxide (CdO); the crystalline form of this compound is a dark red which changes color when heated, similar to zinc oxide. Hydrochloric acid, sulfuric acid, and nitric acid dissolve cadmium by forming cadmium chloride (CdCl2), cadmium sulfate (CdSO4), or cadmium nitrate (Cd(NO3)2). The oxidation state +1 can be produced by dissolving cadmium in a mixture of cadmium chloride and aluminium chloride, forming the Cd22+ cation, which is similar to the Hg22+ cation in mercury(I) chloride.[8]
- Cd + CdCl2 + 2 AlCl3 → Cd2(AlCl4)2
The structures of many cadmium complexes with nucleobases, amino acids, and vitamins have been determined.[11]
Isotopes
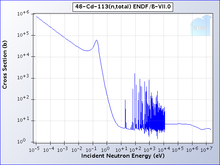
Naturally occurring cadmium is composed of 8 isotopes. Two of them are radioactive, and three are expected to decay but have not done so under laboratory conditions. The two natural radioactive isotopes are 113Cd (beta decay, half-life is 7.7 × 1015 years) and 116Cd (two-neutrino double beta decay, half-life is 2.9 × 1019 years). The other three are 106Cd, 108Cd (both double electron capture), and 114Cd (double beta decay); only lower limits on these half-lives have been determined. At least three isotopes – 110Cd, 111Cd, and 112Cd – are stable. Among the isotopes that do not occur naturally, the most long-lived are 109Cd with a half-life of 462.6 days, and 115Cd with a half-life of 53.46 hours. All of the remaining radioactive isotopes have half-lives of less than 2.5 hours, and the majority have half-lives of less than 5 minutes. Cadmium has 8 known meta states, with the most stable being 113mCd (t1/2 = 14.1 years), 115mCd (t1/2 = 44.6 days), and 117mCd (t1/2 = 3.36 hours).[12]
The known isotopes of cadmium range in atomic mass from 94.950 u (95Cd) to 131.946 u (132Cd). For isotopes lighter than 112 u, the primary decay mode is electron capture and the dominant decay product is element 47 (silver). Heavier isotopes decay mostly through beta emission producing element 49 (indium).[12]
One isotope of cadmium, 113Cd, absorbs neutrons with high selectivity: With very high probability, neutrons with energy below the cadmium cut-off will be absorbed; those higher than the cut-off will be transmitted. The cadmium cut-off is about 0.5 eV, and neutrons below that level are deemed slow neutrons, distinct from intermediate and fast neutrons.[13]
Cadmium is created via the long s-process in low-medium mass stars with masses of 0.6 to 10 solar masses, taking thousands of years.[clarification needed] In that process, a silver atom captures a neutron and then undergoes beta decay.[14]
History

Cadmium (Latin cadmia, Greek καδμεία meaning "calamine", a cadmium-bearing mixture of minerals that was named after the Greek mythological character Κάδμος, Cadmus, the founder of Thebes) was discovered simultaneously in 1817 by Friedrich Stromeyer[15] and Karl Samuel Leberecht Hermann, both in Germany, as an impurity in zinc carbonate.[7] Stromeyer found the new element as an impurity in zinc carbonate (calamine), and, for 100 years, Germany remained the only important producer of the metal. The metal was named after the Latin word for calamine, because it was found in this zinc compound. Stromeyer noted that some impure samples of calamine changed color when heated but pure calamine did not. He was persistent in studying these results and eventually isolated cadmium metal by roasting and reducing the sulfide. The potential for cadmium yellow as pigment was recognized in the 1840s, but the lack of cadmium limited this application.[16][17][18]
Even though cadmium and its compounds are toxic in certain forms and concentrations, the British Pharmaceutical Codex from 1907 states that cadmium iodide was used as a medication to treat "enlarged joints, scrofulous glands, and chilblains".[19]
In 1907, the International Astronomical Union defined the international ångström in terms of a red cadmium spectral line (1 wavelength = 6438.46963 Å).[20][21] This was adopted by the 7th General Conference on Weights and Measures in 1927. In 1960, the definitions of both the metre and ångström were changed to use krypton.[22]
After the industrial scale production of cadmium started in the 1930s and 1940s, the major application of cadmium was the coating of iron and steel to prevent corrosion; in 1944, 62% and in 1956, 59% of the cadmium in the United States was used for plating.[7][23] In 1956, 24% of the cadmium in the United States was used for a second application in red, orange and yellow pigments from sulfides and selenides of cadmium.[23]
The stabilizing effect of cadmium chemicals like the carboxylates cadmium laurate and cadmium stearate on PVC led to an increased use of those compounds in the 1970s and 1980s. The demand for cadmium in pigments, coatings, stabilizers, and alloys declined as a result of environmental and health regulations in the 1980s and 1990s; in 2006, only 7% of to total cadmium consumption was used for plating, and only 10% was used for pigments.[7] At the same time, these decreases in consumption were compensated by a growing demand for cadmium for nickel-cadmium batteries, which accounted for 81% of the cadmium consumption in the United States in 2006.[24]
Occurrence

Cadmium makes up about 0.1 mg kg−1 (ppm) of Earth's crust.[clarification needed] Typical background concentrations in other environmental media are: atmosphere <5 ng m−3; soil <2 mg kg−1; vegetation <0.5 mg kg−1; freshwater <1 ug L−1; seawater <50 ng L−1; sediment <2 mg kg−1.[25] Compared with the more abundant 65 ppm zinc, cadmium is rare.[26] No significant deposits of cadmium-containing ores are known. Greenockite (CdS), the only cadmium mineral of importance, is nearly always associated with sphalerite (ZnS). This association is caused by geochemical similarity between zinc and cadmium, with no geological process likely to separate them. Thus, cadmium is produced mainly as a byproduct from mining, smelting, and refining sulfidic ores of zinc, and, to a lesser degree, lead and copper. Small amounts of cadmium, about 10% of consumption, are produced from secondary sources, mainly from dust generated by recycling iron and steel scrap. Production in the United States began in 1907,[18] but not until after World War I did cadmium come into wide use.[27][28]
Metallic cadmium can be found in the Vilyuy River basin in Siberia.[29]
Rocks mined for phosphate fertilizers contain varying amounts of cadmium, resulting in a cadmium concentration of as much as 300 mg/kg in the fertilizers and a high cadmium content in agricultural soils.[30][31] Coal can contain significant amounts of cadmium, which ends up mostly in flue dust.[32]
Production
The British Geological Survey reports that in 2001, China was the top producer of cadmium with almost one-sixth of the world's production, closely followed by South Korea and Japan.[33]
Cadmium is a common impurity in zinc ores, and it is most often isolated during the production of zinc. Some zinc ores concentrates from sulfidic zinc ores contain up to 1.4% of cadmium.[34] In the 1970s, the output of cadmium was 6.5 pounds per ton of zinc.[34] Zinc sulfide ores are roasted in the presence of oxygen, converting the zinc sulfide to the oxide. Zinc metal is produced either by smelting the oxide with carbon or by electrolysis in sulfuric acid. Cadmium is isolated from the zinc metal by vacuum distillation if the zinc is smelted, or cadmium sulfate is precipitated from the electrolysis solution.[28][35]
-
History of the world production of cadmium
-
Cadmium output in 2005
Applications
Cadmium is a common component of electric batteries, pigments,[36] coatings,[37] and electroplating.[38]
Batteries

In 2009, 86% of cadmium was used in batteries, predominantly in rechargeable nickel-cadmium batteries. Nickel-cadmium cells have a nominal cell potential of 1.2 V. The cell consists of a positive nickel hydroxide electrode and a negative cadmium electrode plate separated by an alkaline electrolyte (potassium hydroxide).[39] The European Union put a limit on cadmium in electronics in 2004 of 0.01%,[40] with some exceptions, and reduced the limit on cadmium content to 0.002%.[41]
Electroplating

Cadmium electroplating, consuming 6% of the global production, is used in the aircraft industry to reduce corrosion of steel components.[38] This coating is passivated by chromate salts.[37] A limitation of cadmium plating is hydrogen embrittlement of high-strength steels from the electroplating process. Therefore, steel parts heat-treated to tensile strength above 1300 MPa (200 ksi) should be coated by an alternative method (such as special low-embrittlement cadmium electroplating processes or physical vapor deposition).
Titanium embrittlement from cadmium-plated tool residues resulted in banishment of those tools (and the implementation of routine tool testing to detect cadmium contamination) in the A-12/SR-71, U-2, and subsequent aircraft programs that use titanium.[42]
Nuclear fission
Cadmium is used in the control rods of nuclear reactors, acting as a very effective "neutron poison" to control neutron flux in nuclear fission.[38] When cadmium rods are inserted in the core of a nuclear reactor, cadmium absorbs neutrons preventing them from creating additional fission events, thus controlling the amount of reactivity. The pressurized water reactor designed by Westinghouse Electric Company uses an alloy consisting of 80% silver, 15% indium, and 5% cadmium.[38]
Compounds

Cadmium oxide was used in black and white television phosphors and in the blue and green phosphors of color television cathode ray tubes.[43] Cadmium sulfide (CdS) is used as a photoconductive surface coating for photocopier drums.[44]

Various cadmium salts are used in paint pigments, with CdS as a yellow pigment being the most common. Cadmium selenide is a red pigment, commonly called cadmium red. To painters who work with the pigment, cadmium provides the most brilliant and durable yellows, oranges, and reds — so much so that during production, these colors are significantly toned down before they are ground with oils and binders or blended into watercolors, gouaches, acrylics, and other paint and pigment formulations. Because these pigments are potentially toxic, users should use a barrier cream on the hands to prevent absorption through the skin[36] even though the amount of cadmium absorbed into the body through the skin is reported to be less than 1%.[9]
In PVC, cadmium was used as heat, light, and weathering stabilizers.[38][45] Currently, cadmium stabilizers have been completely replaced with barium-zinc, calcium-zinc and organo-tin stabilizers. Cadmium is used in many kinds of solder and bearing alloys, because a low coefficient of friction and fatigue resistance.[38] It is also found in some of the lowest-melting alloys, such as Wood's metal.[46]
Laboratory uses

Helium–cadmium lasers are a common source of blue-ultraviolet laser light. They operate at either 325 or 422 nm in fluorescence microscopes and various laboratory experiments.[47][48] Cadmium selenide quantum dots emit bright luminescence under UV excitation (He-Cd laser, for example). The color of this luminescence can be green, yellow or red depending on the particle size. Colloidal solutions of those particles are used for imaging of biological tissues and solutions with a fluorescence microscope.[49]
Cadmium is a component of some compound semiconductors, such as cadmium sulfide, cadmium selenide, and cadmium telluride, used for light detection and solar cells. HgCdTe is sensitive to infrared[38] light and can be used as an infrared detector, motion detector, or switch in remote control devices.
In molecular biology, cadmium is used to block voltage-dependent calcium channels from fluxing calcium ions, as well as in hypoxia research to stimulate proteasome-dependent degradation of Hif-1α.[50]
Cadmium-selective sensors
Cadmium-selective sensors based on the fluorophore BODIPY have been developed for imaging and sensing of cadmium in cells.[51]
Biological role
Cadmium has no known function in higher organisms,[52] but a cadmium-dependent carbonic anhydrase has been found in some marine diatoms.[53] The diatoms live in environments with very low zinc concentrations and cadmium performs the function normally carried out by zinc in other anhydrases. This was discovered with X-ray absorption fluorescence spectroscopy (XAFS).[53][54]
The highest concentration of cadmium is absorbed in the kidneys of humans, and up to about 30 mg of cadmium is commonly inhaled throughout human childhood and adolescence.[55]
Cadmium can be used to block calcium channels in chicken neurons.[56] Analytical methods for the determination of cadmium in biological samples have been reviewed.[57]
Environment
The biogeochemistry of cadmium and its release to the environment has been the subject of review, as has the speciation of cadmium in the environment.[58][59]
Environmental concentrations can exceed adverse-effect-thresholds in cadmium-polluted ecosystems (e.g. in some parts of Europe) and pollutant cadmium can accumulate in invertebrates, earthworms, seabirds, marine mammals, plants, and some algal species; effects in animals include kidney disorders, impairment of enzymes, disruption of calcium metabolism, and changes in cell membrane permeability; excess Cd uptake in plants can affect growth and metabolic processes such as photosynthesis and transpiration.[60]
Safety
The bioinorganic aspects of cadmium toxicity have been reviewed.[61]
The most dangerous form of occupational exposure to cadmium is inhalation of fine dust and fumes, or ingestion of highly soluble cadmium compounds.[7] Inhalation of cadmium fumes can result initially in metal fume fever but may progress to chemical pneumonitis, pulmonary edema, and death.[62]
Cadmium is also an environmental hazard. Human exposure is primarily from fossil fuel combustion, phosphate fertilizers, natural sources, iron and steel production, cement production and related activities, nonferrous metals production, and municipal solid waste incineration.[7] Bread, root crops, and vegetables also contribute to the cadmium in modern populations.[63]
There have been a few instances of general population poisoning as the result of long-term exposure to cadmium in contaminated food and water, and research into an estrogen mimicry that may induce breast cancer is ongoing.[63] In the decades leading up to World War II, mining operations contaminated the Jinzū River in Japan with cadmium and traces of other toxic metals. As a consequence, cadmium accumulated in the rice crops along the riverbanks downstream of the mines. Some members of the local agricultural communities consumed the contaminated rice and developed itai-itai disease and renal abnormalities, including proteinuria and glucosuria.[64]

The victims of this poisoning were almost exclusively post-menopausal women with low iron and other mineral body stores. Similar general population cadmium exposures in other parts of the world have not resulted in the same health problems because the populations maintained sufficient iron and other mineral levels. Thus, although cadmium is a major factor in the itai-itai disease in Japan, most researchers have concluded that it was one of several factors.[7] Cadmium is one of six substances banned by the European Union's Restriction on Hazardous Substances (RoHS) directive, which regulates hazardous substances in electrical and electronic equipment but allows for certain exemptions and exclusions from the scope of the law.[65] The International Agency for Research on Cancer has classified cadmium and cadmium compounds as carcinogenic to humans.[66] Although occupational exposure to cadmium is linked to lung and prostate cancer, there is still a substantial controversy about the carcinogenicity of cadmium in low environmental exposure. Recent data from epidemiological studies suggest that intake of cadmium through diet associates to higher risk of endometrial, breast and prostate cancer as well as to osteoporosis in humans.[67][68][69][70] A recent study has demonstrated that endometrial tissue is characterized by higher levels of cadmium in current and former smoking females.[71]
Cadmium exposure is a risk factor associated with a large number of illnesses including kidney disease,[72] early atherosclerosis, hypertension, and cardiovascular diseases.[73] Although studies show a significant correlation between cadmium exposure and occurrence of disease in human populations, a necessary molecular mechanism has not been identified. One hypothesis holds that cadmium is an endocrine disruptor and some experimental studies have shown that it can interact with different hormonal signaling pathways. For example, cadmium can bind to the estrogen receptor alpha,[74][75] and affect signal transduction along the estrogen and MAPK signaling pathways at low doses.[76][77][78]
Tobacco smoking is the most important single source of cadmium exposure in the general population. An estimated 10% of the cadmium content of a cigarette is inhaled through smoking. Absorption of cadmium through the lungs is more effective than through the gut, and as much as 50% of the cadmium inhaled in cigarette smoke may be absorbed.[79] On average, cadmium concentrations in the blood of smokers is 4 times 5 times greater and in the kidney, 2–3 times greater than non-smokers. Despite the high cadmium content in cigarette smoke, there seems to be little exposure to cadmium from passive smoking.[80]
In a non-smoking population, food is the greatest source of exposure. High quantities of cadmium can be found in crustaceans, mollusks, offal, and algae products. However, grains, vegetables, and starchy roots and tubers are consumed in much greater quantity in the US, and are the source of the greatest dietary exposure.[81] Most plants bio-accumulate metal toxins like Cd, and when composted to form organic fertilizers yield a product which can often contain high amounts (e.g., over 0.5 mg) of metal toxins for every kilo of fertilizer. Fertilizers made from animal dung (e.g., cow dung) or urban waste can contain similar amounts of Cd. The Cd added to the soil from fertilizers (rock phosphates or organic fertilizers) become bio-available and toxic only if the soil pH is low (i.e., acidic soils). Zinc is chemically similar to cadmium and some evidence indicates the presence of Zn ions reduces cadmium toxicity.[82]
Zinc, Cu, Ca, and Fe ions, and selenium with vitamin C are used to treat Cd intoxication, though it is not easily reversed.[72]
Regulations
Because of the adverse effects of cadmium on the environment and human health, the supply and use of cadmium is restricted in Europe under the REACH Regulation.[83]
The EFSA Panel on Contaminants in the Food Chain specifies that 2.5 μg/kg body weight is a tolerable weekly intake for humans.[81] The Joint FAO/WHO Expert Committee on Food Additives has declared 7 μg/kg bw to be the provisional tolerable weekly intake level.[84]
The US Occupational Safety and Health Administration (OSHA) has set the permissible exposure limit (PEL) for cadmium at a time-weighted average (TWA) of 0.005 ppm. The National Institute for Occupational Safety and Health (NIOSH) has not set a recommended exposure limit (REL) and has designated cadmium as a known human carcinogen. The IDLH (immediately dangerous to life and health) level for cadmium is 9 mg/m3.[85]
| Lethal dose[86] | Organism | Route | Time |
|---|---|---|---|
| LD50: 225 mg/kg | rat | oral | n/a |
| LD50: 890 mg/kg | mouse | oral | n/a |
| LC50: 25 mg/m3 | rat | n/a | 30 min |
Product recalls
In May 2006, a sale of the seats from Arsenal F.C.'s old stadium, Highbury in London, England was cancelled when the seats were discovered to contain trace amounts of cadmium.[87] Reports of high levels of cadmium use in children's jewelry in 2010 led to a US Consumer Product Safety Commission investigation.[88] The U.S. CPSC issued specific recall notices for cadmium content in jewelry sold by Claire's[89] and Wal-Mart[90] stores.
In June 2010, McDonald's voluntarily recalled more than 12 million promotional "Shrek Forever After 3D" Collectable Drinking Glasses because of the cadmium levels in paint pigments on the glassware.[91] The glasses were manufactured by Arc International, of Millville, NJ, USA.[92]
See also
References
- ^ "Standard Atomic Weights: Cadmium". CIAAW. 2013.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (4 May 2022). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c d Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ a b c d e f g Morrow, H. (2010). "Cadmium and Cadmium Alloys". Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons. pp. 1–36. doi:10.1002/0471238961.0301041303011818.a01.pub3. ISBN 978-0-471-23896-6.
- ^ a b Holleman, A. F.; Wiberg, E; Wiberg, Nils (1985). "Cadmium". Lehrbuch der Anorganischen Chemie, 91–100 (in German). Walter de Gruyter. pp. 1056–1057. ISBN 978-3-11-007511-3.
- ^ a b "Case Studies in Environmental Medicine (CSEM) Cadmium". Agency for Toxic Substances and Disease Registry. Archived from the original on 6 June 2011. Retrieved 30 May 2011.
- ^ Cotton, F. A. (1999). "Survey of Transition-Metal Chemistry". Advanced Inorganic Chemistry (6th ed.). John Wiley and Sons. p. 633. ISBN 0-471-19957-5.
- ^ Carballo, Rosa; Castiñeras, Alfonso; Domínguez-Martin, Alicia; García-Santos, Isabel; Niclós-Guttiérrez, Juan (2013). "Chapter 7. Solid state structures of cadmium complexes with relevance to biological systems". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 145–189. doi:10.1007/978-94-007-5179-8_7.
- ^ a b Audi, G.; Bersillon, O.; Blachot, J.; Wapstra, A.H. (2003). "The NUBASE Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729 (1): 3–128. Bibcode:2003NuPhA.729....3A. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ Knoll, G. F. (2000). Radiation Detection and Measurement. Wiley. p. 505. ISBN 978-0-471-07338-3.
- ^ Padmanabhan, T. (2001). "Stellar Nucleosynthesis". Theoretical Astrophysics, Volume II: Stars and Stellar Systems. Cambridge University Press. pp. 230–236. ISBN 978-0-521-56631-5.
- ^ Hermann, C. S. (1818). "Noch ein schreiben über das neue Metall". Annalen der Physik. 59 (5): 113–116. Bibcode:1818AnP....59..113H. doi:10.1002/andp.18180590511.
- ^ Waterston, W.; Burton, J. H (1844). Cyclopædia of commerce, mercantile law, finance, commercial geography and navigation. H. G. Bohn. p. 122.
- ^ Rowbotham, T.; Rowbotham, T. L. (1850). The Art of Landscape Painting in Water Colours. Windsor and Newton. p. 10.
- ^ a b Ayres, R. U.; Ayres, L.; Råde, I. (2003). The Life Cycle of Copper, Its Co-Products and Byproducts. Springer. pp. 135–141. ISBN 978-1-4020-1552-6.
- ^ Dunglison, R. (1866). Medical Lexicon: A Dictionary of Medical Science. Henry C. Lea. p. 159.
- ^ "International Angstrom". Science Dictionary. 14 September 2013. Retrieved 24 September 2014.
- ^ "angstrom or ångström". Sizes.com. 28 October 2010. Retrieved 24 September 2014.
- ^ Burdun, G. D. (1958). "On the new determination of the meter" (PDF). Measurement Techniques. 1 (3): 259–264. doi:10.1007/BF00974680.
- ^ a b Lansche, A. M. (1956). "Cadmium". Minerals Yearbook, Volume I: Metals and Minerals (Except Fuels). United States Geological Survey. Retrieved 21 April 2008.
- ^ "USGS Mineral Information: Cadmium". United States Geological Survey. Retrieved 8 August 2009.
- ^ Rieuwerts, J. [1], The Elements of Environmental Pollution, Routledge, Abingdon and New York, 2015.
- ^ Wedepohl, K. H. (1995). "The composition of the continental crust". Geochimica et Cosmochimica Acta. 59 (7): 1217–1232. Bibcode:1995GeCoA..59.1217W. doi:10.1016/0016-7037(95)00038-2.
- ^ Plachy, J. (1998). "Annual Average Cadmium Price" (PDF). U.S. Geological Survey. pp. 17–19. Retrieved 16 June 2010.
- ^ a b Fthenakis, V. M. (2004). "Life cycle impact analysis of cadmium in CdTe PV production". Renewable and Sustainable Energy Reviews. 8 (4): 303–334. doi:10.1016/j.rser.2003.12.001.
- ^ Fleischer, M; Cabri, L. J.; Chao, G. Y.; Pabst, A. (1980). "New Mineral Names" (PDF). American Mineralogist. 65: 1065–1070.
- ^ Grant, C. A.; Sheppard, S. C. (2008). "Fertilizer impacts on cadmium availability in agricultural soils and crops". Human and Ecological Risk Assessment. 14 (2): 210–228. doi:10.1080/10807030801934895.
- ^ Jiao, Y.; Grant, C. A.; Bailey, L. D. (2004). "Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat". Journal of the Science of Food and Agriculture. 84 (8): 777–785. doi:10.1002/jsfa.1648.
- ^ Bettinelli, M.; Baroni, U.; Pastorelli, N. (1988). "Determination of arsenic, cadmium, lead, antimony, selenium and thallium in coal fly ash using the stabilised temperature platform furnace and Zeeman-effect background correction". Journal of Analytical Atomic Spectrometry. 3 (7): 1005–1011. doi:10.1039/JA9880301005.
- ^ Hetherington, L. E.; et al. (2008). "Production of Cadmium". World Mineral Production 2002–06 (PDF). British Geological Survey. p. 15. Retrieved 15 April 2012.
- ^ a b Golberg, D. C.; et al. (1969). Trends in Usage of Cadmium: Report. US NRC/NAS/NAE. pp. 1–3.
- ^ Scoullos, M. J. (2001). Mercury, Cadmium, Lead: Handbook for Sustainable Heavy Metals Policy and Regulation. Springer. pp. 104–116. ISBN 978-1-4020-0224-3.
- ^ a b Buxbaum, Gunter; Pfaff, Gerhard (2005). "Cadmium Pigments". Industrial inorganic pigments. Wiley-VCH. pp. 121–123. ISBN 978-3-527-30363-2.
- ^ a b Smith C.J.E.; Higgs M.S.; Baldwin K.R. (20 April 1999). "Advances to Protective Coatings and their Application to Ageing Aircraft". RTO MP-25. Archived from the original (PDF) on 17 May 2011. Retrieved 29 May 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g Scoullos, Michael J.; Vonkeman, Gerrit H.; Thornton, Iain; Makuch, Zen (2001). Mercury, Cadmium, Lead: Handbook for Sustainable Heavy Metals Policy and Regulation. Springer. ISBN 978-1-4020-0224-3.
- ^ Krishnamurthy, N (2 July 2013). Engg. Chemistry, 2/e. New York: PHI Learning Private Limited. pp. 82–83. ISBN 978-81-203-3666-7.
- ^ http://eur-lex.europa.eu/legal-content/en/ALL/;jsessionid=VN9WTn1VHCtTbGnQpnkrTsj2gh68QzJf581nbhhVpJSLNn2sLJsy!-561601488?uri=CELEX:32011L0065
- ^ [2]
- ^ "CIA – Breaking Through Technological Barriers – Finding The Right Metal (A-12 program)". 1 October 2007.
- ^ Lee, Ching-Hwa; Hsi, CS (2002). "Recycling of Scrap Cathode Ray Tubes". Environmental Science & Technology. 36 (1): 69–75. Bibcode:2002EnST...36...69L. doi:10.1021/es010517q. PMID 11811492.
- ^ Miller, L. S.; Mullin, J. B. (1991). "Crystalline Cadmium Sulfide". Electronic materials: from silicon to organics. Springer. p. 273. ISBN 978-0-306-43655-0.
- ^ Jennings, Thomas C. (2005). "Cadmium Environmental Concerns". PVC handbook. Hanser Verlag. p. 149. ISBN 978-1-56990-379-7.
- ^ Brady, George Stuart; Brady, George S.; Clauser, Henry R.; Vaccari, John A. (2002). Materials handbook: an encyclopedia for managers, technical professionals, purchasing and production managers, technicians, and supervisors. McGraw-Hill Professional. p. 425. ISBN 978-0-07-136076-0.
- ^ "Helium-Cadmium Lasers". Olympus. Retrieved 14 May 2011.
- ^ Nambiar, K.R (2006). "Helium-cadmium Laser". Lasers: Principles, Types and Applications. ISBN 978-81-224-1492-9.
- ^ "Cadmium Selenium Testing for Microbial Contaminants". NASA. 10 June 2003.
- ^ Park J. W., Chun Y. S.; Choi, E; Kim, G. T.; Choi, H.; Kim, C. H.; Lee, M. J.; Kim, M. S.; Park, J. W. (2000). "Cadmium blocks hypoxia-inducible factor (HIF)-1-mediated response to hypoxia by stimulating the proteasome-dependent degradation of HIF-1alpha". European Journal of Biochemistry. 267 (13): 4198–4204. doi:10.1046/j.1432-1327.2000.01453.x. PMID 10866824.
- ^ Taki, Masayasu (2013). "Chapter 5. Imaging and sensing of cadmium in cells". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. p. 99115. doi:10.1007/978-94-007-5179-8_5.
- ^ Hogan, C. Michael (2010). Heavy metal. Encyclopedia of Earth. National Council for Science and the Environment. E. Monosson and C. Cleveland (eds.). Washington DC.
- ^ a b Lane, Todd W.; Saito, Mak A.; George, Graham N.; Pickering, Ingrid J.; Prince, Roger C.; Morel, François M. M. (2005). "A cadmium enzyme from a marine diatom" (PDF). Nature. 435 (42): 42. Bibcode:2005Natur.435...42L. doi:10.1038/435042a. PMID 15875011.
- ^ Lane, Todd W.; Morel, F. M. (2000). "A biological function for cadmium in marine diatoms". Proc. Natl. Acad. Sci. 97 (9): 4627–4631. Bibcode:2000PNAS...97.4627L. doi:10.1073/pnas.090091397. PMC 18283. PMID 10781068.
- ^ Perry, HM Jr.; Thind, GS; Perry, EF (1976). "The biology of cadmium". The Medical clinics of North America. 60 (4): 759–69. PMID 775217.
- ^ Swandulla, D.; Armstrong, C. M. (1989). "Calcium channel block by cadmium in chicken sensory neurons" (PDF). Proc. Natl. Acad. Sci. 86 (5): 1736–1740. Bibcode:1989PNAS...86.1736S. doi:10.1073/pnas.86.5.1736. PMC 286776. PMID 2537985.
- ^ Klotz, Katrin; Weistenhöfer, Wobbeke; Drexler, Hans (2013). "Chapter 4. Determination of Cadmium in Biological Samples". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 85–98. doi:10.1007/978-94-007-5179-8_4.
- ^ Cullen, Jay T.; Maldonado, Maria T. (2013). "Chapter 2. Biogeochemistry of Cadmium and its Release to the Environment". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 31–62. doi:10.1007/978-94-007-5179-8_2.
- ^ Crea, Francesco; Foti, Claudia; Milea, Demetrio; Sammartano, Silvio (2013). "Chapter 3. Speciation of Cadmium in the Environment". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 63–83. doi:10.1007/978-94-007-5179-8_3.
- ^ Rieuwerts, J (2015). [3], The Elements of Environmental Pollution, Routledge, Abingdon and New York.
- ^ Maret, Wolfgang; Moulis, Jean-Marc (2013). "Chapter 1. The Bioinorganic Chemistry of Cadmium in the Context of its Toxicity". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 1–30. doi:10.1007/978-94-007-5179-8_1.
- ^ Hayes, Andrew Wallace (2007). Principles and Methods of Toxicology. Philadelphia: CRC Press. pp. 858–861. ISBN 978-0-8493-3778-9.
- ^ a b Mann, Denise (23 April 2012) Can Heavy Metal in Foods, Cosmetics Spur Breast Cancer Spread? HealthDayBy via Yahoo
- ^ Nogawa, Koji; Kobayashi, E; Okubo, Y; Suwazono, Y (2004). "Environmental cadmium exposure, adverse effects, and preventative measures in Japan". Biometals. 17 (5): 581–587. doi:10.1023/B:BIOM.0000045742.81440.9c. PMID 15688869.
- ^ "European Commission Decision of 12 October 2006 amending, for the purposes of adapting to technical progress, the Annex to Directive 2002/95/EC of the European Parliament and of the Council as regards exemptions for applications of lead and cadmium (notified under document number C(2006) 4790)". Journal of the European Union. 14 October 2006.
- ^ IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 58
- ^ Julin, B; Wolk, A; Johansson, J. E.; Andersson, S. O.; Andrén, O; Akesson, A (2012). "Dietary cadmium exposure and prostate cancer incidence: A population-based prospective cohort study". British Journal of Cancer. 107 (5): 895–900. doi:10.1038/bjc.2012.311. PMC 3425979. PMID 22850555.
- ^ Engström, A; Michaëlsson, K; Vahter, M; Julin, B; Wolk, A; Åkesson, A (2012). "Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women". Bone. 50 (6): 1372–8. doi:10.1016/j.bone.2012.03.018. PMID 22465267.
- ^ Julin, B; Wolk, A; Bergkvist, L; Bottai, M; Akesson, A (2012). "Dietary cadmium exposure and risk of postmenopausal breast cancer: A population-based prospective cohort study". Cancer Research. 72 (6): 1459–66. doi:10.1158/0008-5472.CAN-11-0735. PMID 22422990.
- ^ Akesson, A; Julin, B; Wolk, A (2008). "Long-term dietary cadmium intake and postmenopausal endometrial cancer incidence: A population-based prospective cohort study". Cancer Research. 68 (15): 6435–41. doi:10.1158/0008-5472.CAN-08-0329. PMID 18676869.
- ^ Rzymski, P; Rzymski, P; Tomczyk, K; Niedzielski, P; Jakubowski, K; Poniedziałek, B; Opala, T (2014). "Metal status in human endometrium: Relation to cigarette smoking and histological lesions". Environmental Research. 132: 328–33. Bibcode:2014ER....132..328R. doi:10.1016/j.envres.2014.04.025. PMID 24834829.
- ^ a b http://www.arltma.com/Articles/CadmiumToxDoc.htm
- ^ Cadmium Exposure can Induce Early Atherosclerotic Changes, Medinews Direct, 7 September 2009
- ^ Fechner, P; Damdimopoulou, P; Gauglitz, G (2011). "Biosensors paving the way to understanding the interaction between cadmium and the estrogen receptor alpha". PLOS ONE. 6 (8): e23048. Bibcode:2011PLoSO...623048F. doi:10.1371/journal.pone.0023048. PMC 3149063. PMID 21829690.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stoica, A; Katzenellenbogen, B. S.; Martin, M. B. (2000). "Activation of estrogen receptor-alpha by the heavy metal cadmium". Molecular endocrinology (Baltimore, Md.). 14 (4): 545–53. doi:10.1210/mend.14.4.0441. PMID 10770491.
- ^ Ali, I; Penttinen-Damdimopoulou, P. E.; Mäkelä, S. I.; Berglund, M; Stenius, U; Akesson, A; Håkansson, H; Halldin, K (2010). "Estrogen-like effects of cadmium in vivo do not appear to be mediated via the classical estrogen receptor transcriptional pathway". Environmental Health Perspectives. 118 (10): 1389–94. doi:10.1289/ehp.1001967. PMC 2957917. PMID 20525538.
- ^ Ali, I; Damdimopoulou, P; Stenius, U; Adamsson, A; Mäkelä, S. I.; Åkesson, A; Berglund, M; Håkansson, H; Halldin, K (2012). "Cadmium-induced effects on cellular signaling pathways in the liver of transgenic estrogen reporter mice". Toxicological Sciences. 127 (1): 66–75. doi:10.1093/toxsci/kfs077. PMID 22314386.
- ^ Johnson, M. D.; Kenney, N; Stoica, A; Hilakivi-Clarke, L; Singh, B; Chepko, G; Clarke, R; Sholler, P. F.; Lirio, A. A.; Foss, C; Reiter, R; Trock, B; Paik, S; Martin, M. B. (2003). "Cadmium mimics the in vivo effects of estrogen in the uterus and mammary gland". Nature Medicine. 9 (8): 1081–4. doi:10.1038/nm902. PMID 12858169.
- ^ Friberg, L. (1983). "Cadmium". Annual Review of Public Health. 4: 367–367. doi:10.1146/annurev.pu.04.050183.002055. PMID 6860444.
- ^ Jarup, L. (1998). "Health effects of cadmium exposure—a review of the literature and a risk estimate". Scandinavian Journal of Work, Environment and Health. 24: 11–51.
- ^ a b http://www.efsa.europa.eu/en/efsajournal/pub/2551.htm
- ^ http://innovareacademics.in/journals/index.php/ijpps/article/view/2305
- ^ EUR-Lex. Eur-lex.europa.eu (18 April 2011). Retrieved on 5 June 2011.
- ^ http://www.inchem.org/documents/jecfa/jeceval/jec_297.htm
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0087". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Cadmium compounds (as Cd)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ "Toxic fears hit Highbury auction". BBC Sport. 10 May 2006. Retrieved 29 November 2010.
- ^ "U.S. to Develop Safety Standards for Toxic Metals". Business Week. 12 January 2010. Retrieved 12 January 2010.
- ^ "Claire's Recalls Children's Metal Charm Bracelets Due to High Levels of Cadmium". U.S. Consumer Product Safety Commission. 10 May 2010. Retrieved 5 June 2010.
- ^ "FAF Inc. Recalls Children's Necklaces Sold Exclusively at Walmart Stores Due to High Levels of Cadmium". U.S. Consumer Product Safety Commission. 29 January 2010. Retrieved 5 June 2010.
- ^ Neuman, William (4 June 2010). "McDonald's Recalls 12 Million 'Shrek' Glasses". The New York Times. Retrieved 5 June 2010.
- ^ "McDonald's Recalls Movie Themed Drinking Glasses Due to Potential Cadmium Risk". U.S. Consumer Product Safety Commission. 4 June 2010. Retrieved 5 June 2010.
Further reading
- Hartwig, Andrea (2013). "Chapter 15. Cadmium and cancer". In Astrid Sigel; Helmut Sigel; Roland K. O. Sigel (eds.). Cadmium: From Toxicology to Essentiality. Metal Ions in Life Sciences. Vol. 11. Springer. pp. 491–507. doi:10.1007/978-94-007-5179-8_15.
- Rieuwerts, John (2015). "Chapter 8. Cadmium". The Elements of Environmental Pollution. Abingdon, UK: Routledge. ISBN 9780415859202.
{{cite book}}: CS1 maint: ref duplicates default (link)
External links
- Cadium at The Periodic Table of Videos (University of Nottingham)
- ATSDR Case Studies in Environmental Medicine: Cadmium Toxicity U.S. Department of Health and Human Services
- Agency for Toxic Substances and Disease Registry’s (ATSDR) Toxicological Profile for Cadmium
- National Institute for Occupational Safety and Health – Cadmium Page
- NLM Hazardous Substances Databank – Cadmium, Elemental
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).



