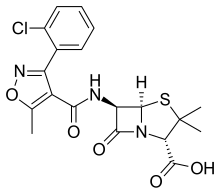
Cloxacillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Cloxapen, others |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Routes of administration | By mouth, IM |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 37 to 90% |
| Protein binding | 95% |
| Elimination half-life | 30 minutes to 1 hour |
| Excretion | kidney and biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.468 |
| Chemical and physical data | |
| Formula | C19H18ClN3O5S |
| Molar mass | 435.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cloxacillin is an antibiotic useful for the treatment of several bacterial infections.[1] This includes impetigo, cellulitis, pneumonia, septic arthritis, and otitis externa.[1] It is not effective for methicillin-resistant Staphylococcus aureus (MRSA).[2] It can be used by mouth and by injection.[1]
Side effects include nausea, diarrhea, and allergic reactions including anaphylaxis.[1] Clostridioides difficile diarrhea may also occur.[2] It is not recommended in people who have previously had a penicillin allergy.[1] Use during pregnancy appears to be relatively safe.[1] Cloxacillin is in the penicillin family of medications.[2]
Cloxacillin was patented in 1960 and approved for medical use in 1965.[3] It is on the World Health Organization's List of Essential Medicines.[4] It is not commercially available in the United States.[2]
Mechanism of action
[edit]It is semisynthetic and in the same class as penicillin. Cloxacillin is used against staphylococci that produce beta-lactamase, due to its large R chain, which does not allow the beta-lactamases to bind. This drug has a weaker antibacterial activity than benzylpenicillin, and is devoid of serious toxicity except for allergic reactions.[citation needed]
Society and culture
[edit]Cloxacillin was discovered and developed by Beecham (now GlaxoSmithKline).[5]
It is sold under a number of trade names, including Cloxapen, Cloxacap, Tegopen and Orbenin.[6]
See also
[edit]References
[edit]- ^ a b c d e f World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. pp. 98, 100, 110–111, 586, 602, 614, 623. hdl:10665/44053. ISBN 9789241547659.
- ^ a b c d "Cloxacillin (Professional Patient Advice)". www.drugs.com. Archived from the original on 20 December 2016. Retrieved 10 December 2016.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Greenwood D (2008). Antimicrobial drugs: chronicle of a twentieth century medical triumph. Oxford University Press US. pp. 124–. ISBN 978-0-19-953484-5. Archived from the original on 6 June 2013. Retrieved 18 November 2010.
- ^ Gollakner R (2023-05-09). "Cloxacillin". VCA Animal Hospitals. Retrieved 2023-05-09.
External links
[edit]- "Cloxacillin". Drug Information Portal. U.S. National Library of Medicine.
