Emodin
| |

| |
| Names | |
|---|---|
| IUPAC name
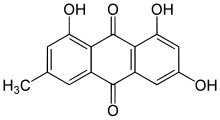
1,3,8-Trihydroxy-6-methylanthracene-9,10-dione
| |
| Other names
6-Methyl-1,3,8-trihydroxyanthraquinone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.509 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C15H10O5 | |
| Molar mass | 270.240 g·mol−1 |
| Appearance | Orange solid[1] |
| Density | 1.583±0.06 g/cm3 |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Emodin (6-methyl-1,3,8-trihydroxyanthraquinone) is a chemical compound that can be isolated from rhubarb, buckthorn, and Japanese knotweed (Reynoutria japonica syn. Polygonum cuspidatum).[2] It is specifically isolated from Rheum Palmatum L[3]. It is also produced by many species of fungi, including members of the genera Aspergillus, Pyrenochaeta, and Pestalotiopsis, inter alia. The common name is derived from Rheum emodi, a taxonomic synonym of Rheum australe, (Himalayan rhubarb) and synonyms include emodol, frangula emodin, rheum emodin, 3-methyl-1,6,8-trihydroxyanthraquinone, Schuttgelb, and Persian Berry Lake.[4]
List of plant species
The following plant species produce emodin:
- Acalypha australis[5]
- Cassia occidentalis[6]
- Cassia siamea[7]
- Frangula alnus[8]
- Glossostemon bruguieri [9]
- Kalimeris indica[10]
- Polygonum hypoleucum[11]
- Reynoutria japonica (syn. Fallopia japonica)[12] (syn. Polygonum cuspidatum[13])
- Rhamnus alnifolia, the alderleaf buckthorn[14]
- Rhamnus cathartica, the common buckthorn[14]
- Rheum palmatum[15]
- Rumex nepalensis[16]
- Senna obtusifolia[17] (syn. Cassia obtusifolia[18])
- Thielavia subthermophila[19]
- Ventilago madraspatana[20]
Compendial status
References
- ^ Herbal Extract Online. http://herbalextractonline.com/Herbal-Extract/Emodin.html (accessed 9 November 2014).
- ^ Dorland's Medical Dictionary (1938)
- ^ Palaniyandi, Karyppaiya. Medicinal Plants - Recent Advances in Research and Development. Singapore : Springer Singapore : Imprint: Springer. p. 339. ISBN 978-981-10-5978-0. Retrieved 11/15/2019.
{{cite book}}: Check date values in:|accessdate=(help) - ^ CID 3220 from PubChem
- ^ Wang, X. L.; Yu, K. B.; Peng, S. L. (2008). "[Chemical constituents of aerial part of Acalypha australis]" [Chemical Constituents of Aerial Part of Acalypha australis]. Zhongguo Zhong Yao Za Zhi [China Journal of Chinese Materia Medica] (in Chinese). 33 (12): 1415–1417. PMID 18837345.
- ^ Yadav, J. P.; Arya, V.; Yadav, S.; Panghal, M.; Kumar, S.; Dhankhar, S. (2010). "Cassia occidentalis L.: A Review on its Ethnobotany, Phytochemical and Pharmacological Profile". Fitoterapia. 81 (4): 223–230. doi:10.1016/j.fitote.2009.09.008. PMID 19796670.
- ^ Nsonde Ntandou, G. F.; Banzouzi, J. T.; Mbatchi, B.; Elion-Itou, R. D.; Etou-Ossibi, A. W.; Ramos, S.; Benoit-Vical, F.; Abena, A. A.; Ouamba, J. M. (2010). "Analgesic and Anti-Inflammatory Effects of Cassia siamea Lam. Stem Bark Extracts". Journal of Ethnopharmacology. 127 (1): 108–111. doi:10.1016/j.jep.2009.09.040. PMID 19799981.
- ^ Faculty of Pharmacy and Biochemistry, University of Zagreb, A. Kovačića 1, 10000 Zagreb, Croatia b Dipartimento di Scienze del Farmaco, Università degli Studi “G. d’Annunzio” di Chieti-Pescara, Via dei Vestini 31, 66100 Chieti, Italy (April 2012). "Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark". Food Chemistry. 131 (4): 1174–1180. doi:10.1016/j.foodchem.2011.09.094.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: numeric names: authors list (link) - ^ http://www.mdpi.net/molecules/papers/80800614.pdf
- ^ Wang, G.; Wang, G. K.; Liu, J. S.; Yu, B.; Wang, F.; Liu, J. K. (2010). "[Studies on the chemical constituents of Kalimeris indica]" [Studies on the Chemical Constituents of Kalimeris indica]. Zhong Yao Cai (in Chinese). 33 (4): 551–554. PMID 20845783.
- ^ Chao, P. M.; Kuo, Y. H.; Lin, Y. S.; Chen, C. H.; Chen, S. W.; Kuo, Y. H. (2010). "The Metabolic Benefits of Polygonum hypoleucum Ohwi in HepG2 Cells and Wistar Rats under Lipogenic Stress". Journal of Agricultural and Food Chemistry. 58 (8): 5174–5180. doi:10.1021/jf100046h. PMID 20230058.
- ^ "Archived copy". Archived from the original on 16 June 2013. Retrieved 3 May 2011.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Ban, S. H.; Kwon, Y. R.; Pandit, S.; Lee, Y. S.; Yi, H. K.; Jeon, J. G. (2010). "Effects of a Bio-Assay Guided Fraction from Polygonum cuspidatum Root on the Viability, Acid Production and Glucosyltranferase of mutans streptococci". Fitoterapia. 81 (1): 30–34. doi:10.1016/j.fitote.2009.06.019. PMID 19616082.
- ^ a b Sacerdote, Allison B.; King, Richard B. (2014). "Direct Effects of an Invasive European Buckthorn Metabolite on Embryo Survival and Development in Xenopus laevis and Pseudacris triseriata" (PDF). Journal of Herpetology. 48 (1): 51–58. doi:10.1670/12-066.
- ^ Liu, A.; Chen, H.; Wei, W.; Ye, S.; Liao, W.; Gong, J.; Jiang, Z.; Wang, L.; Lin, S. (2011). "Antiproliferative and Antimetastatic Effects of Emodin on Human Pancreatic Cancer". Oncology Reports. 26 (1): 81–89. doi:10.3892/or.2011.1257. PMID 21491088.
- ^ Gautam, R.; Karkhile, K. V.; Bhutani, K. K.; Jachak, S. M. (2010). "Anti-Inflammatory, Cyclooxygenase (COX)-2, COX-1 Inhibitory, and Free Radical Scavenging Effects of Rumex nepalensis". Planta Medica. 76 (14): 1564–1569. doi:10.1055/s-0030-1249779. PMID 20379952.
- ^ Dr. Duke's Phytochemical and Ethnobotanical Databases
- ^ Yang, Y.-C.; Lim, M.-Y.; Lee, H.-S. (2003). "Emodin Isolated from Cassia obtusifolia (Leguminosae) Seed Shows Larvicidal Activity against Three Mosquito Species". Journal of Agricultural and Food Chemistry. 51 (26): 7629–7631. doi:10.1021/jf034727t. PMID 14664519.
- ^ Kusari, S.; Zühlke, S.; Košuth, J.; Čellárová, E.; Spiteller, M. (2009). "Light-Independent Metabolomics of Endophytic Thielavia subthermophila Provides Insight into Microbial Hypericin Biosynthesis". Journal of Natural Products. 72 (10): 1825–1835. doi:10.1021/np9002977. PMID 19746917.
- ^ Ghosh, S.; Das Sarma, M.; Patra, A.; Hazra, B. (2010). "Anti-Inflammatory and Anticancer Compounds Isolated from Ventilago madraspatana Gaertn., Rubia cordifolia Linn. and Lantana camara Linn". Journal of Pharmacy and Pharmacology. 62 (9): 1158–1166. doi:10.1111/j.2042-7158.2010.01151.x. PMID 20796195.
- ^ The British Pharmacopoeia Secretariat (2009). "Index, BP 2009" (PDF). Archived from the original (PDF) on 11 April 2009. Retrieved 20 April 2010.
