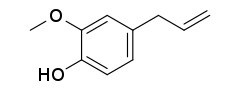
Eugenol
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-Methoxy-4-(prop-2-en-1-yl)phenol | |
| Other names
4-Allyl-2-methoxyphenol
2-Methoxy-4-(2-propenyl)phenol Eugenic acid Caryophyllic acid 1-Allyl-3-methoxy-4-hydroxybenzene Allylguaiacol 2-Methoxy-4-allylphenol 4-Allylcatechol-2-methyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.355 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.204 g·mol−1 |
| Density | 1.06 g/cm3 |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 254 °C (489 °F; 527 K) |
| Acidity (pKa) | 10.19 at 25 °C |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 104 °C (219 °F; 377 K) |
| Related compounds | |
Related compounds
|
2-Phenethyl propionate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Eugenol /juːɡɛnɒl/ is a phenylpropene, an allyl chain-substituted guaiacol. Eugenol is a member of the phenylpropanoids class of chemical compounds. It is a colourless to pale yellow oily liquid extracted from certain essential oils especially from clove oil, nutmeg, cinnamon, basil and bay leaf.[1][2][3][4] It is present in concentrations of 80–90% in clove bud oil and at 82–88% in clove leaf oil.[5]
Modern uses
Eugenol is used in perfumes, flavorings, and essential oils. It is also used as a local antiseptic and anaesthetic.[6][unreliable medical source?] Eugenol can be combined with zinc oxide to form zinc oxide eugenol which has restorative and prosthodontic applications in dentistry. For example, zinc oxide eugenol is used for root canal sealing.[7]
Attempts have been made to develop eugenol derivatives as intravenous anesthetics, as an alternative to propanidid which produces unacceptable side effects around the site of injection in many patients.[8]
It can be used to reduce the presence of Listeria monocytogenes and Lactobacillus sakei in food.[9]
It is also used in manufacturing stabilizers and antioxidants for plastics and rubbers.
It is one of many compounds that is attractive to males of various species of orchid bees, which apparently gather the chemical to synthesize pheromones; it is commonly used as bait to attract and collect these bees for study.[10] It also attracts female cucumber beetle.[11] It was recently discovered that eugenol and isoeugenol, floral volatile scent compounds, are catalyzed by a single type of enzyme in the Gymnadenia species and gene encoding for this enzyme is the first functionally characterized gene in this species so far.[12]
Clove oil is growing in popularity as an anaesthetic for use on aquarium fish as well as on wild fish when sampled for research and management purposes.[13][14] Where readily available, it presents a humane method to euthanise sick and diseased fish either by direct over-dose or to induce sleep before an overdose of ethanol.[15]
It is also used in some mousetraps.[citation needed]
Toxicity
Eugenol is hepatotoxic, meaning it may cause damage to the liver.[16][17] Overdose is possible, causing a wide range of symptoms from blood in the patient's urine, to convulsions, diarrhoea, nausea, unconsciousness, dizziness, or rapid heartbeat.[18] According to a published 1993 report, a 2-year-old boy nearly died after taking between 5 and 10 ml[19] In context, this would represent a toxic dose in the range of 500-1000mg/kg, approximately one third that of table salt.
Allergy
Eugenol is subject to restrictions on its use in perfumery[20] as some people may become sensitised to it, however, the degree to which eugenol can cause an allergic reaction in humans is disputed.[21]
Eugenol is a component of Balsam of Peru, to which some people are allergic.[22][23] When eugenol is used in dental preparations such as surgical pastes, dental packing, and dental cement, it may cause contact stomatitis and allergic cheilitis.[22] The allergy can be discovered via a patch test.[22]
Natural occurrence
Eugenol is naturally occurring in several plants, including the following:
- Cloves (Syzygium aromaticum)[24][25][26]
- Wormwood
- Cinnamon[25][27]
- Cinnamomum tamala[28]
- Nutmeg (Myristica fragrans)[29]
- Ocimum basilicum[30] – Sweet Basil
- Ocimum gratissimum[12][31] – African Basil
- Ocimum tenuiflorum (Ocimum sanctum) – Tulsi or Holy Basil
- Japanese star anise[32]
- Lemon balm[33]
- Dill
- Pimenta racemosa
- Vanilla
- Bay laurel
- Celery
See also
References
- ^ "Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston" (PDF). Bangladesh Council of Scientific and Industrial Research BCSIR Laboratories. 4: 451–454.
- ^ Mallavarapu, Gopal R.; Ramesh, S.; Chandrasekhara, R. S.; Rajeswara Rao, B. R.; Kaul, P. N.; Bhattacharya, A. K. (1995). "Investigation of the essential oil of cinnamon leaf grown at Bangalore and Hyderabad". Flavour and Fragrance Journal. 10 (4). wiley.com: 239–242. doi:10.1002/ffj.2730100403. Retrieved 27 April 2014.
- ^ Yield and Oil Composition of 38 Basil (Ocimum basilicum L.) Accessions Grown in Mississippi Archived 15 October 2010 at the Wayback Machine
- ^ "Typical G.C. for bay leaf oil". Thegoodscentscompany.com. Retrieved 27 April 2014.
- ^ Barnes, J; Anderson, LA; Phillipson, JD (2007) [1996]. Herbal Medicines (PDF) (3rd ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85369-623-0.
- ^ Jadhav BK, Khandelwal KR, Ketkar AR, Pisal SS.; Khandelwal; Ketkar; Pisal (February 2004). "Formulation and evaluation of mucoadhesive tablets containing eugenol for the treatment of periodontal diseases". Drug Dev Ind Pharm. 30 (2): 195–203. doi:10.1081/DDC-120028715. PMID 15089054.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jack L. Ferracane (2001). Materials in Dentistry: Principles and Applications (2nd ed.). Lippincott Williams & Wilkins. ISBN 0-7817-2733-2.
- ^ Right DA, Payne JP; Payne (June 1962). "A clinical study of intravenous anaesthesia with a eugenol derivative, G.29.505" (abstract). British Journal of Anaesthesia. 34 (6): 379–385. doi:10.1093/bja/34.6.379. PMID 14008420.
- ^ Gill, A. O.; Holley, R. A. (2004). "Mechanisms of Bactericidal Action of Cinnamaldehyde against Listeria monocytogenes and of Eugenol against L. Monocytogenes and Lactobacillus sakei". Applied and Environmental Microbiology. 70 (10): 5750–5. doi:10.1128/AEM.70.10.5750-5755.2004. PMC 522076. PMID 15466510.
- ^ Schiestl FP, Roubik DW; Roubik (January 2003). "Odor Compound Detection in Male Euglossine Bees". Journal of Chemical Ecology. 29 (1): 253–257. doi:10.1023/A:1021932131526. PMID 12647866.
- ^ "Cucumber Beetles: Organic and Biorational Integrated Pest Management (Summary)". Attra.ncat.org. 5 August 2013. Retrieved 27 April 2014.
- ^ a b Gupta AK, Schauvinhold I, Pichersky E, Schiestl FP (2014). "Eugenol synthase genes in floral scent variation in Gymnadenia species". Functional & Integrative Genomics. 14 (4): 779–88. doi:10.1007/s10142-014-0397-9. PMID 25239559.
- ^ Anesthesia, Analgesia, and Surgery in Pet Fish. Atlantic Coast Veterinary Conference. 2001. Retrieved 17 March 2014.
- ^ Grush, J; Noakes, DLG; Moccia, RD (February 2004). "The Efficacy of Clove Oil As An Anesthetic for the Zebrafish". Zebrafish. 1 (1): 46–53. doi:10.1089/154585404774101671. PMID 18248205.
- ^ Monks, Neale (2 April 2009). "Aquarium Fish Euthanasia" (PDF). Fish Channel. Retrieved 7 December 2010.
- ^ Thompson, DC; Barhoumi, R; Burghardt, RC (1998). "Comparative toxicity of eugenol and its quinone methide metabolite in cultured liver cells using kinetic fluorescence bioassays". Toxicology and applied pharmacology. 149 (1): 55–63. doi:10.1006/taap.1997.8348. PMID 9512727.
- ^ Fujisawa, S; Atsumi, T; Kadoma, Y; Sakagami, H (2002). "Antioxidant and prooxidant action of eugenol-related compounds and their cytotoxicity". Toxicology. 177 (1): 39–54. doi:10.1016/S0300-483X(02)00194-4. PMID 12126794.
- ^ Eugenol Oil Overdose, New York Times Health Guide
- ^ Hartnoll, G; Moore, D; Douek, D (1993). "Near fatal ingestion of oil of cloves". Archives of Disease in Childhood. 69 (3): 392–3. doi:10.1136/adc.69.3.392. PMC 1029532. PMID 8215554.
- ^ www.ifraorg.org Archived 30 December 2011 at the Wayback Machine
- ^ . www.leffingwell.com http://www.leffingwell.com/Cropwatch%20Claims%20Victory%20Over%2026%20Allergens.pdf. Retrieved 27 April 2014.
{{cite web}}:|url=missing title (help) - ^ a b c Gottfried Schmalz; Dorthe Arenholt Bindslev (10 October 2008). Biocompatibility of Dental Materials. ISBN 9783540777823. Retrieved 27 April 2014.
- ^ Derk P. Bruynzeel (2014). "Management of Positive Patch Test Reactions". Springer: 53–55. doi:10.1007/978-3-642-55706-4_11. ISBN 978-3-540-44347-6. Retrieved 27 April 2014.
{{cite journal}}:|chapter=ignored (help); Cite journal requires|journal=(help) - ^ Pathak, SB; Niranjan, K; Padh, H; Rajani, M (2004). "TLC Densitometric Method for the Quantification of Eugenol and Gallic Acid in Clove". Chromatographia. 60 (3–4): 241–244. doi:10.1365/s10337-004-0373-y.
- ^ a b Bullerman, LB; Lieu, FY; Seier, SA (July 1977). "INHIBITION OF GROWTH AND AFLATOXIN PRODUCTION BY CINNAMON AND CLOVE OILS. CINNAMIC ALDEHYDE AND EUGENOL". Journal of Food Science. 42 (4): 1107–1109. doi:10.1111/j.1365-2621.1977.tb12677.x.
- ^ Lee, Kwang-Geun; Takayuki Shibamoto (2001). "Antioxidant property of aroma extract isolated from clove buds [Syzygium aromaticum (L.) Merr. et Perry]". Food Chemistry. 74 (4): 443–448. doi:10.1016/S0308-8146(01)00161-3.
- ^ Kreydiyyeh, SI; Usta, J; Copti, Rtitle = Effect of cinnamon, clove and some of their constituents on the Na+-K+-ATPase activity and alanine absorption in the rat jejunum (2000). "Effect of cinnamon, clove and some of their constituents on the Na(+)-K(+)-ATPase activity and alanine absorption in the rat jejunum". Food and Chemical Toxicology. 38 (9): 755–762. doi:10.1016/S0278-6915(00)00073-9. PMID 10930696.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dighe, VV; Gursale, AA; Sane, RT; Menon, S; Patel, PH (2005). "Quantitative Determination of Eugenol from Cinnamomum tamala Nees and Eberm. Leaf Powder and Polyherbal Formulation Using Reverse Phase Liquid Chromatography". Chromatographia. 61 (9–10): 443–446. doi:10.1365/s10337-005-0527-6.
- ^ Bennett, A; Stamford, IF; Tavares, IA; Jacobs, S; Capasso, F; Mascolo, N; Autore, G; Romano, V; Di Carlo, G (1988). "The biological activity of eugenol, a major constituent of nutmeg (Myristica fragrans): Studies on prostaglandins, the intestine and other tissues". Phytotherapy Research. 2 (3): 124–130. doi:10.1002/ptr.2650020305.
- ^ Johnson, CB; Kirby, J; Naxakis, G; Pearson, S (1999). "Substantial UV-B-mediated induction of essential oils in sweet basil (Ocimum basilicum L.)". Phytochemistry. 51 (4): 507–510. doi:10.1016/S0031-9422(98)00767-5.
- ^ Nakamura, CV; Ueda-Nakamura, T; Bando, E; Melo, AFN; Cortez, DAG; Dias Filho, BP (September 1999). "Antibacterial activity of Ocimum gratissimum L. essential oil" (PDF). Memórias do Instituto Oswaldo Cruz. 94 (5): 675–678. doi:10.1590/S0074-02761999000500022. PMID 10464416.
- ^ Ize-Ludlow, D; Ragone, S; Bruck, IS; Bernstein, JN; Duchowny, M; Peña, BM (2004). "Neurotoxicities in Infants Seen With the Consumption of Star Anise Tea". Pediatrics. 114 (5): e653–e656. doi:10.1542/peds.2004-0058. PMID 15492355.
- ^ "Lemon balm". University of Maryland Medical Center. Retrieved 7 December 2010.
External links
- Kegley, SE; Hill, BR; Orme S; Choi AH; Toxicity Information for Eugenol in PAN Pesticide Database, Pesticide Action Network, North America (San Francisco, CA, 2010)
- "Eugenol (Mmm, dentist) : Molecule of the Day". ScienceBlogs. 4 October 2006. Retrieved 7 December 2010. (Eugenol entry on the Molecule of the Day blog)

