Myelin basic protein
| Myelin_MBP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Myelin_MBP | ||||||||
| Pfam | PF01669 | ||||||||
| InterPro | IPR000548 | ||||||||
| PROSITE | PDOC00492 | ||||||||
| SCOP2 | 1bx2 / SCOPe / SUPFAM | ||||||||
| OPM superfamily | 464 | ||||||||
| OPM protein | 2lug | ||||||||
| |||||||||
Myelin basic protein (MBP) is a protein believed to be important in the process of myelination of nerves in the nervous system. The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the velocity of axonal impulse conduction.[5] MBP maintains the correct structure of myelin, interacting with the lipids in the myelin membrane.[6][7]
MBP was initially sequenced in 1971 after isolation from myelin membranes.[8] Since that time, knockout mice deficient in MBP that showed decreased amounts of CNS myelination and a progressive disorder characterized by tremors, seizures, and early death have been developed. The human gene for MBP is on chromosome 18;[9] the protein localizes to the CNS and to various cells of the hematopoietic system.
The pool of MBP in the central nervous system is very diverse, with several splice variants being expressed and a large number of post-translational modifications on the protein, which include phosphorylation, methylation, deamidation, and citrullination. These forms differ by the presence or the absence of short (10 to 20 residues) peptides in various internal locations in the sequence. In general, the major form of MBP is a protein of about 18.5 Kd (170 residues).
In melanocytic cell types, MBP gene expression may be regulated by MITF.[10]
Gene expression
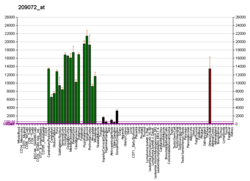
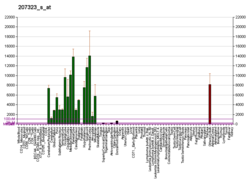
The protein encoded by the classic MBP gene is a major constituent of the myelin sheath of oligodendrocytes and Schwann cells in the nervous system. However, MBP-related transcripts are also present in the bone marrow and the immune system. These mRNAs arise from the long MBP gene (otherwise called "Golli-MBP") that contains 3 additional exons located upstream of the classic MBP exons. Alternative splicing from the Golli and the MBP transcription start sites gives rise to 2 sets of MBP-related transcripts and gene products. The Golli mRNAs contain 3 exons unique to Golli-MBP, spliced in-frame to 1 or more MBP exons. They encode hybrid proteins that have N-terminal Golli aa sequence linked to MBP aa sequence. The second family of transcripts contain only MBP exons and produce the well-characterized myelin basic proteins. This complex gene structure is conserved among species, suggesting that the MBP transcription unit is an integral part of the Golli transcription unit and that this arrangement is important for the function and/or regulation of these genes.[11]
Role in disease
Interest in MBP has centered on its role in demyelinating diseases, in particular, multiple sclerosis (MS). Several studies have shown a role for antibodies against MBP in the pathogenesis of MS.[12] Some studies have linked a genetic predisposition to MS to the MBP gene, though a majority have not.
Some recent works have shown that inoculating an animal with MBP to generate an immune response against it increases blood–brain barrier permeability.[citation needed]
A targeted immune response to MBP has been researched in lethal rabies infection. The inoculation of MBP generates increases the permeability of the blood–brain barrier (BBB), allowing immune cells to enter the brain, the primary site of rabies virus replication. In a study of mice infected with Silver-haired bat rabies virus (SHBRV), the mortality rate of mice treated with MBP improved 20%-30% over the untreated control group. It is significant to note that healthy uninfected mice treated with MBP showed an increase in mortality rate between 0% and 40%.[13]
A "molecular mimicry" hypothesis of multiple sclerosis has been suggested, in which T cells are, in essence, confusing MBP with human herpesvirus-6. Researchers in the United States created a synthetic peptide with a sequence identical to that of an HHV-6 peptide. They were able to show that T cells were activated by this peptide. These activated T cells also recognized and initiated an immune response against a synthetically created peptide sequence that is identical to part of human MBP. During their research, they found that the levels of these cross-reactive T cells are significantly elevated in multiple sclerosis patients.[14]
Interactions
Myelin basic protein has been shown to interact in vivo with Proteolipid protein 1,[15][16] and in vitro with Calmodulin, Actin, Tropomyosin, Tubulin, Clathrin, 2',3'-Cyclic-nucleotide 3'-phosphodiesterase and multiple molecules of the Immune system.[17]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000197971 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000041607 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Sakamoto Y, Kitamura K, Yoshimura K, Nishijima T, Uyemura K (March 1987). "Complete amino acid sequence of PO protein in bovine peripheral nerve myelin". J. Biol. Chem. 262 (9): 4208–14. PMID 2435734.
- ^ Deber CM, Reynolds SJ (April 1991). "Central nervous system myelin: structure, function, and pathology". Clin. Biochem. 24 (2): 113–34. doi:10.1016/0009-9120(91)90421-a. PMID 1710177.
- ^ Inouye H, Kirschner DA (January 1991). "Folding and function of the myelin proteins from primary sequence data". J. Neurosci. Res. 28 (1): 1–17. doi:10.1002/jnr.490280102. PMID 1710279.
- ^ Eylar EH, Brostoff S, Hashim G, Caccam J, Burnett P (September 1971). "Basic A1 protein of the myelin membrane. The complete amino acid sequence". J. Biol. Chem. 246 (18): 5770–84. PMID 5096093.
- ^ Saxe DF, Takahashi N, Hood L, Simon MI (1985). "Localization of the human myelin basic protein gene (MBP) to region 18q22----qter by in situ hybridization". Cytogenet. Cell Genet. 39 (4): 246–9. doi:10.1159/000132152. PMID 2414074.
- ^ Hoek KS, Schlegel NC, Eichhoff OM, Widmer DS, Praetorius C, Einarsson SO, Valgeirsdottir S, Bergsteinsdottir K, Schepsky A, Dummer R, Steingrimsson E (December 2008). "Novel MITF targets identified using a two-step DNA microarray strategy". Pigment Cell Melanoma Res. 21 (6): 665–76. doi:10.1111/j.1755-148X.2008.00505.x. PMID 19067971.
- ^ "Entrez Gene: myelin basic protein".
- ^ Berger T, Rubner P, Schautzer F, Egg R, Ulmer H, Mayringer I, Dilitz E, Deisenhammer F, Reindl M (July 2003). "Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event". N. Engl. J. Med. 349 (2): 139–45. doi:10.1056/NEJMoa022328. PMID 12853586.
- ^ Roy A, Hooper DC (August 2007). "Lethal silver-haired bat rabies virus infection can be prevented by opening the blood–brain barrier". J. Virol. 81 (15): 7993–8. doi:10.1128/JVI.00710-07. PMC 1951307. PMID 17507463.
- ^ Tejada-Simon, Maria (2003). "Cross-reactivity with myelin basic protein and human herpesvirus-6 in multiple sclerosi". Annals of Neurology. 53 (2): 189–97. doi:10.1002/ana.10425. PMID 12557285.
- ^ Wood DD, Vella GJ, Moscarello MA (October 1984). "Interaction between human myelin basic protein and lipophilin". Neurochem. Res. 9 (10): 1523–31. doi:10.1007/BF00964678. PMID 6083474.
- ^ Edwards AM, Ross NW, Ulmer JB, Braun PE (January 1989). "Interaction of myelin basic protein and proteolipid protein". J. Neurosci. Res. 22 (1): 97–102. doi:10.1002/jnr.490220113. PMID 2467009.
- ^ Harauz, George; et al. (2004). "Myelin basic protein—diverse conformational states of an intrinsically unstructured protein and its roles in myelin assembly and multiple sclerosis". Micron. 35 (7): 503–542. doi:10.1016/j.micron.2004.04.005.
Further reading
- Shanshiashvili L, Mikeladze D (2003). "Some aspects of myelin basic protein". J. Biol. Phys. Chem. 3: 96–9. doi:10.4024/18SH03R.jbpc.03.03.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.