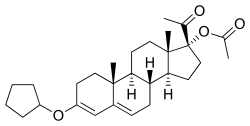
Pentagestrone acetate
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Gestovis, Gestovister |
| Other names | 17α-Acetoxyprogesterone 3-cyclopentyl enol ether |
| Routes of administration | By mouth |
| Drug class | Progestin; Progestogen; Progestogen ether; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C28H40O4 |
| Molar mass | 440.615 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pentagestrone acetate, sold under the brand names Gestovis and Gestovister and also known as 17α-acetoxyprogesterone 3-cyclopentyl enol ether, is a progestin of the 17α-hydroxyprogesterone group which was described in the literature in 1960 and was introduced by Vister in Italy in 1961.[1][2][3] It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate.[4] Pentagestrone acetate, along with quingestrone (the 3-cyclopentyl enol ether of progesterone), is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.[5]
Chemistry
See also
References
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 943–. ISBN 978-1-4757-2085-3.
- ^ Drugs Available Abroad. Gale Research. 1991. ISBN 978-0-8103-7177-4.
- ^ P. H. List; L. Hörhammer (12 March 2013). Chemikalien und Drogen Teil A: N-Q. Springer-Verlag. pp. 508–. ISBN 978-3-642-65035-2.
- ^ Camille Georges Wermuth (2 May 2011). The Practice of Medicinal Chemistry. Academic Press. pp. 731–. ISBN 978-0-08-056877-5.
- ^ Revue générale des sciences pures et appliquées et bulletin de l'Association française pour l'avancement des sciences. Société d'édition d'enseignement supérieur. 1964.
[[...] Ercoli (1960) developed cyclopentyl enol ethers of progesterone (Luteovis) and acetoxy progesterone (Gestovis), which have almost exactly the same properties as dydrogesterone.]
