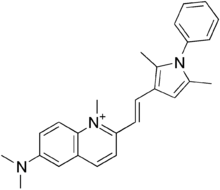
Pyrvinium
Appearance
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.020.543 |
| Chemical and physical data | |
| Formula | C26H28N3+ |
| Molar mass | 382.52 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pyrvinium (Viprynium) is an anthelmintic effective for pinworms.[1] Several forms of pyrvinium have been prepared with variable counter anions, such as halides, tosylate, triflate and pamoate.[2][3]
Pyrvinium salts can also inhibit the growth of cancer cells.[4] More specifically, the pamoate salt has been shown to have preferential toxicity for various cancer cell lines during glucose starvation.[5]
Synthesis
One synthetic method is based on Skraup synthesis and Paal-Knorr synthesis.[4] More recently, an alternative convergent, synthetic strategy to pyrvinium triflate salts through Friedländer synthesis was reported.[3]
References
- ^ Desai, A. S. (1962). "Single-dose treatment of oxyuriasis with pyrvinium embonate". British Medical Journal. 2 (5319): 1583–1585. doi:10.1136/bmj.2.5319.1583. PMC 1926864. PMID 14027194.
- ^ PubChem: Pyrvinium
- ^ a b An, Jing; Mao, Yongjun; Lin, Nan; Tian, Wang; Huang, Ziwei (2012). "New Synthesis of Pyrvinium That inhibits the β-Catenin/Tcf4 Pathway". Heterocycles. 85 (5): 1179–1185. doi:10.3987/COM-12-12446.
- ^ a b WO 2006078754, J. E. Macdonald, M. K. Hysell, D. Yu, H. Li, and F. Wong-Staal, "Novel Quinolinium Salts and Derivatives", published 2006-07-27
- ^ Esumi, H.; Lu, J.; Kurashima, Y.; Hanaoka, T. (2004). "Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl-1-methyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation". Cancer science. 95 (8): 685–690. doi:10.1111/j.1349-7006.2004.tb03330.x. PMID 15298733.
