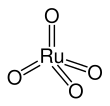
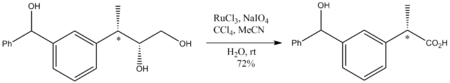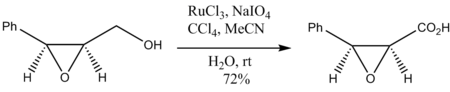Ruthenium tetroxide
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ruthenium(VIII) oxide
| |||
| Identifiers | |||
| ECHA InfoCard | 100.039.815 | ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| RuO4 | |||
| Molar mass | 165.07 g/mol | ||
| Appearance | colorless liquid | ||
| Odor | pungent | ||
| Density | 3.29 g/cm3 | ||
| Melting point | 25.4 °C (77.7 °F; 298.5 K) | ||
| Boiling point | 40.0 °C (104.0 °F; 313.1 K) | ||
| 2% w/v at 20 °C | |||
| Solubility in other solvents | Soluble in Carbon tetrachloride Chloroform | ||
| Structure | |||
| tetrahedral | |||
| zero | |||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Related compounds | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Ruthenium tetroxide (Ruthenium(VIII) oxide) is the inorganic compound with the formula RuO4. It is a colourless liquid but samples are typically black due to impurities. As expected for a charge-neutral symmetrical oxide, it is quite volatile. The analogous OsO4 is more widely used and better known. One of the few solvents in which it forms stable solutions is CCl4.
Preparation
RuO4 is prepared by oxidation of ruthenium(III) chloride with NaIO4.
- 8 Ru3+(aq) + 5 IO4−(aq) + 12 H2O(l) → 8 RuO4(s) + 5 I−(aq) + 24 H+(aq)
In typical reactions featuring RuO4 as the oxidant, many forms of ruthenium usefully serve as precursors to RuO4, such as oxide hydrates or hydrated chloride.
Structure
The molecule adopts tetrahedral. The Ru-O distances range from 169 to 170 pm.[1]
RuO4 is diamagnetic.
It oxidizes virtually any hydrocarbon. For example, it will oxidize adamantane to 1-adamantanol. It is used in organic synthesis to oxidize terminal alkynes to 1,2-diketones and primary alcohols to carboxylic acids. When used in this fashion, the ruthenium(VIII) oxide is used in catalytic amounts and regenerated by the addition of sodium periodate to ruthenium(III) chloride and a solvent mixture of acetonitrile, water and carbon tetrachloride.
Because it is such an aggressive oxidant, reaction conditions are mild, generally room temperature. Although a strong oxidant, RuO4 oxidations do not perturb stereocenters that are not oxidized. Illustrative is the oxidation of the following diol to a carboxylic acid:
Oxidation of epoxy alcohols also occurs without degradation of the epoxide ring:
Under milder condition, oxidative reaction yields aldehydes instead. RuO4 readily converts secondary alcohols into ketones. Although similar results can be achieved with other cheaper oxidants such as PCC- or DMSO-based oxidants, RuO4 is ideal when a very vigorous oxidant is needed but mild conditions must be maintained.
RuO4 readily cleaves double bonds to yield carbonyl products, in a manner similar to ozonolysis. Osmium(VIII) oxide, a more familiar oxidant that is structurally similar to RuO4, does not cleave double bonds, instead producing vicinal diol products.
In terms of practical details, the substrate to be oxidized is typically dissolved in solvent such as CCl4, and acetonitrile is added as an aiding ligand to the catalytic cycle. Ether can then be added to precipitate and recover the ruthenium pre-catalyst.
Ruthenium tetroxide is used to expose latent fingerprints by turning to the brown/black ruthenium dioxide when in contact with fatty oils or fats contained in sebaceous contaminants of the print. [2]
Oxidative catalyst and mechanism
Although used as a direct oxidant, due to the relatively high cost of RuO4 it is also used catalytically with a cooxidant. For an oxidation of cyclic alcohols with RuO4 as a catalyst and bromate as a base, RuO4 is first activated by hydroxide:
- RuO4 + OH− → HRuO5−
The reaction proceeds via a glycolate complex.
Related ruthenium compounds
Because RuO4 will readily decompose explosively at slightly elevated temperatures, most laboratories do not synthesize it directly, nor is it commercially available. Most laboratories instead use the anionic Ru(VII) derivative in the form of the salt of "TPAP" (tetrapropylammonium perruthenate), [N(C3H7)4]RuO4. TPAP is synthesized by oxidizing RuCl3 to RuO4− by NaBrO3 and isolated as the tetrapropylamine cation, which allows the salt to be used in organic solvents.
References
- ^ Pley, M.; Wickleder, M. S. (2005). "Two Crystalline Modifications of RuO4". Journal of Solid State Chemistry. 178 (10): 3206–3209. doi:10.1016/j.jssc.2005.07.021.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mashiko, K.; Miyamoto, T. (1998). "Latent Fingerprint Processing by the Ruthenium Tetroxide Method". Journal of Forensic Identification. 48 (3): 279–290. doi:10.3408/jasti.2.21.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Cotton, S.A. (1997). Chemistry of Precious Metals. London: Chapman and Hall. ISBN 978-0-7514-0413-5.
- Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1002/047084289X.rr009.pub2, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1002/047084289X.rr009.pub2instead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1039/B109851A, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1039/B109851Ainstead. - Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1007/BF01129466, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1007/BF01129466instead. - Courtney, J.L.; Swansbor, K.F. (1972). "Ruthenium tetroxide oxidation". Reviews of Pure and Applied Chemistry. 22: 47.