Wikipedia:Reference desk/Archives/Science/2009 March 25
| Science desk | ||
|---|---|---|
| < March 24 | << Feb | March | Apr >> | March 26 > |
| Welcome to the Wikipedia Science Reference Desk Archives |
|---|
| The page you are currently viewing is an archive page. While you can leave answers for any questions shown below, please ask new questions on one of the current reference desk pages. |
March 25
[edit]Radioactive sickness?
[edit]I am writing a research report on radioactive sickness. But when I search on Google I also get radiation sickness in my resaults. Is radioactive sickness and radiation sickness the same thing? —Preceding unsigned comment added by 174.6.144.211 (talk) 00:59, 25 March 2009 (UTC)
- I've never heard of "radioactive sickness". The illness caused by exposure to ionising radiation is called "radiation sickness". What do you mean by "radioactive sickness"? --Tango (talk) 01:07, 25 March 2009 (UTC)
I got a topic on Aleksandr Litvinenko's poisoning and was asked to write a report on radioactive poisoning, as this is what happened to him. But, as i mentioned before, i am not sure whether or not radioactive poisoning and radiation poisoning is the same thing, sine they both pop up when i search for radioactive poisoning. —Preceding unsigned comment added by 174.6.144.211 (talk) 01:12, 25 March 2009 (UTC)
- Litvinenko died of radiation poisoning. I think whoever it was that said "radioactive poisoning" just make a mistake. --Tango (talk) 01:27, 25 March 2009 (UTC)
- All these phrases are talking about the same thing, but "radiation sickness" is the proper name for it. It's not really a kind of poisoning, although people talk of it that way. (Poisons injure the body in different ways than radiation does.) --Anonymous, 01:32 UTC, March 25, 2009.
- Or acute radiation syndrome if you want to sound really clever! --Tango (talk) 01:33, 25 March 2009 (UTC)
- All these phrases are talking about the same thing, but "radiation sickness" is the proper name for it. It's not really a kind of poisoning, although people talk of it that way. (Poisons injure the body in different ways than radiation does.) --Anonymous, 01:32 UTC, March 25, 2009.
(after multiple edit conflicts) We have an article on this incident, Alexander Litvinenko poisoning. The name for what he died from is radiation poisoning. It is surmised that this was caused by the ingestion of a radioactive substance, rather than from exposure to an external source of radiation. One could call it "radioactive poisoning" in that he was poisoned by a radioactive substance as opposed to a toxic chemical such as cyanide or a neurotoxin of some sort. However, there is no real difference between what killed him and what killed the victims of the Chernobyl disaster; the only distinction is the means by which he was exposed to the lethal dose of ionizing radiation. - EronTalk 01:34, 25 March 2009 (UTC)
Wait, so the affect of external radiation does not count as radioactive poisoning? —Preceding unsigned comment added by 174.6.144.211 (talk) 01:51, 25 March 2009 (UTC)
- Yes, it does. It doesn't matter where the radiation comes from or how it gets to your tissues, the effect is still called radiation poisoning/sickness. --Tango (talk) 01:55, 25 March 2009 (UTC)
- Yes - my earlier response may have added some confusion here. I was speculating as to why someone might call what happened to Litvinenko "radioactive poisoning" - I didn't mean to infer any difference between internal and external sources of radiation. - EronTalk 02:26, 25 March 2009 (UTC)
Can radioactive poisoning be genetic? —Preceding unsigned comment added by 174.6.144.211 (talk) 02:10, 25 March 2009 (UTC)
- No. Radiation poisoning (not "radioactive poisoning" - that is not the proper name for it) is caused by exposure to high levels of ionizing radiation. There is no genetic component to it. - EronTalk 02:28, 25 March 2009 (UTC)
Does nuclear warfare, nuclear reactors, radioactive materials and gamma rays cause radiation poisoning because they realease ionizing radiation? —Preceding unsigned comment added by 174.6.144.211 (talk) 02:36, 25 March 2009 (UTC)
- I wrote a good chunk of the Alexander Litvinenko poisoning article - it's an amazing story, the closest thing to a classic Hollywood spy story you'll ever see in real life. But what was significant here is that Polonium-210 (which is the radioactive material that was mixed into Litvinenko's tea - and which eventually killed him) - is not normally particularly dangerous stuff. In fact, you can buy significant quantities of it in anti-static lens cleaning equipment from any decent camera store. It's radioactive - but only emits alpha radiation - which is stopped by a single sheet of paper. Even if you get Polonium-210 directly on your skin - the layer of dead skin cells that covers your body is quite enough to stop the radiation from harming you. What's dangerous is only if you eat (or in this case, drink) the stuff (or perhaps breathe it in as a fine dust in large enough quantities). That spreads it throughout your body and gives the alpha radiation a way to irradiate living tissue. I suspect that is the reason why everyone calls it a 'poisoning' - it's not at all like standing a foot away from a chunk of plutonium and getting irradiated. It only took a couple of days for Litvinenko to show symptoms bad enough to put him in hospital - but it was close to three weeks until he eventually died. For the majority of that time, it was thought that he had been poisoned with non-radioactive Thallium - because although his symptoms were classic symptoms of radiation sickness (nausea, hair falling out, that kind of thing) - there was no measurable radiation coming from his body. So they looked for more conventional poisons that produce that set of symptoms, and thallium popped up. But again - this is probably why the term "poisoning" was initially kicked around and kinda stuck - even after it was realised that this wasn't strictly a poison. The thing that's particularly chilling about this is that the murderer could have used any of a dozen - much easier to obtain - poisons in Litvinenko's tea. This one is so unique and trivially easy to trace (it has to be made in a nuclear reactor - and there are only two sources of it - one Russian and another American - but the isotope ratios make it abundantly clear that this is batch is Russian - and that it was made very soon before the poisoning took place (suggesting that the stuff hadn't been smuggled through long, complicated chains of black-market dealers). The Russians aren't stupid - they'd have known that this particular technique for killing him would be instantly traceable back to them. So it's clear that the point of the exercise was to send a message: "You aren't safe from us anywhere - and we don't care that anyone out there knows that we do this kind of thing."...that's real cold-war stuff! What we don't know is who, specifically, this rather gruesome message was intended for...whoever that is - is evidently keeping a much lower profile! Anyway - enjoy reading the article - and be sure to follow the links to the 150 or so references at the bottom - there is much more to be learned here! SteveBaker (talk) 05:03, 25 March 2009 (UTC)
- (Nitpick: Plutonium is not that radioactive—a lot less so than Polonium-210. You won't get irradiated from standing a foot away from a chunk of it assuming the chunk is not a critical mass. As with Polonium, it is a radiological danger primarily as an alpha emitter that you might inhale. Eating it isn't even really a problem—it passes out of the system rather quickly—it is only a serious problem if it gets into your lungs or bones. Also I would dispute that Polonium-210 is not "normally dangerous stuff"—the amount in anti-static brushes is quite small. You don't need much of it, in terms of mass, to have a problem. It is far more toxic than uranium, for example. It is not as bad as a fission product. But it is still stuff to handle with care in any significant amount.) --140.247.240.69 (talk) 18:43, 25 March 2009 (UTC)
- The claim that Polonium-210 is present in 'significant quantities' in antistatic brushes is somewhat misleading. The largest brush sold by these guys has about 500 microCuries of Polonium, which according to our article is about a tenth of a microgram. That's more than enough to kill you if you decide to eat it, but is nonetheless a pretty small amount. If you actually had a decent-sized chunk of it (a gram, say) on your skin, then your initial problem would be the way the intense heat was burning through your skin. Algebraist 11:17, 26 March 2009 (UTC)
Did Litvinenko suffer from chronic radiation syndrome or acute radiation syndrome? and why? —Preceding unsigned comment added by 174.6.144.211 (talk) 00:33, 27 March 2009 (UTC)
- Acute. He didn't live long enough to develop any chronic problems. Algebraist 00:40, 27 March 2009 (UTC)
What is the difference between chronic and acute problems? —Preceding unsigned comment added by 174.6.144.211 (talk) 01:22, 27 March 2009 (UTC)
- See Acute (medicine) and Chronic (medicine). Algebraist 01:25, 27 March 2009 (UTC)
- (ec) wikt:chronic, wikt:acute. Chronic are long-term problems, acute are short-term. The common cold is generally an acute problem, asthma is generally a chronic one. --Tango (talk) 01:28, 27 March 2009 (UTC)
So, in other words, the concequences are acute radiation syndrome are short-term and the concequences for chronic radiation syndrome are long-tern? —Preceding unsigned comment added by 174.6.144.211 (talk) 01:34, 27 March 2009 (UTC)
- Yes. So after the Chernobyl disaster - the amazingly heroic guys who worked in the core of the reactor to put out the fire died within days of acute radiation sickness. The people in the towns and cities further away from the reactor didn't die from the immediate consequences - but instead have lifetime increases in cancer risk, birth deformities and so forth. That's a chronic consequence. Litvinenko lived for just short of three weeks - but his fate was sealed from the moment he drank the polonium-laced tea - so that's acute radiation sickness. SteveBaker (talk) 02:05, 27 March 2009 (UTC)
- And it's a very important distinction! Often people get the two things very much mixed up—different things cause each of them, generally. The thing about radiation is that something is either VERY radioactive but has a SHORT half-life, or it is WEAKLY radioactive but has a LONG half-life. So polonium has a half-life of only a few months—that's pretty radioactive, as things go, but not as bad as, say, fission products (raw radioactive waste), which will kill you within minutes of close exposure. On the other hand, something like, say, plutonium, has a very long half-life, meaning it won't kill you from close exposure, but sticks around a long time. If during part of that long time it happens to be inside, say, your lungs or bones, it'll sit there radiating and radiating, doing lots of long-term, chronic damage. BOTH of these kinds of radiation risks are bad but they are bad for different reasons and caused by different things. Thus when the Civil Defense guys say you can come out of your fallout shelter after a few days, what they mean is, the stuff that is going to ACUTELY kill you has already radiated itself out of existence by that point. But the long-term, CHRONIC stuff is going to be around for thousands of years, proving a real long-term hazard as part of the food chain, building materials, etc. Just because something is weakly radioactive does not make it safe at all — it just means it won't kill you immediately, but can still kill you in, say, 10-20 years! This is something that even very savvy scientific types often lose sight of (and we've had lots and lots of incidents with this—Chernobyl, Castle Bravo, uranium miners, etc.—where the physicists jump in and say, "oh, it's pretty safe, it's weakly radioactive only, nobody seems to have died!" and then only decades later the health problems show up and everybody gets cancer). --98.217.14.211 (talk) 19:12, 29 March 2009 (UTC)
In Flight Pitch Control / Dive or Stall
[edit]Can someone with knowledge of the principles of flight help me understand something? A UK company named Parajet is building a flying car of sorts called the SkyCar. At the link the above, they make the following statement about the SkyCar:
"It has no pitch control and therefore (is) impossible to stall or dive."
So what is it about having pitch control in fight that makes stalling or diving possible? Why would its absence prevent these situations? Appreciate any input. Wolfgangus (talk) 01:23, 25 March 2009 (UTC)
- "Pitch control" means being able to control what angle the nose is at in a vertical direction. Obviously, that is required to dive - diving is pointing to nose steeply downwards. Stalling is caused by trying to climb too steeply (or not descending fast enough if you're going quite slowly). Presumably this flying car sets its own pitch, somehow, at a level somewhere below where it would stall. (I guess you then control altitude by varying speed - slow down to go down, speed up to go up. However, that would mean it must be possible to either stall, or dive - if you cut the engines, one of those two things has to happen.)--Tango (talk) 01:32, 25 March 2009 (UTC)
- I imagine they just mean it is possible for you to cause a dive or stall by applying too much upward or downward pitch. --Anonymous, 21:35 UTC, March 25, 2009.
- Actually, I take my last statement back - cutting the engines wouldn't be sufficient, you would probably need some way to actually brake to get slow enough for the only non-stalling configuration to be a dive. --Tango (talk) 01:36, 25 March 2009 (UTC)
- I imagine they just mean it is possible for you to cause a dive or stall by applying too much upward or downward pitch. --Anonymous, 21:35 UTC, March 25, 2009.
- What stalls a plane is when the pitch of the wing to the airflow is too steep. If you pitch the plane up and have plenty of engine power - the plane climbs and the airflow remaines pretty much parallel to the wing....but if you pitch the plane up without enough power applied - you get into a vicious circle where the wing loses lift - you start to fall downwards - which means that the airflow is now somewhat upwards - which further increases the angle of pitch to the airflow - which increases the drag - which slows you down - and the pitch angle to the airflow increases still further - until you're simply falling like a rock. What's suspicious about this claim is that even without pitch control - if you slow the motor down enough then the roughly 4 degrees of upward pitch (which you pretty much have to have built into the wing to make the plane fly) - is enough to cause a low-speed stall. The way you recover from such a stall is to push the nose down and (if you can) you apply power. Without pitch control - if your engine fails - you've got no means to recover from the stall and you're going to crash.
- There is a means to fix this problem - and that's to have a 'canard' design - where the pitch control happens on surfaces in front of the main wing and with a slightly steeper pitch than the main wing. What happens then is that if your speed drops, the little 'canard' wings stall before the main wing can - when the front wings stall - they lose lift - the nose falls - and that automatically reduces the angle of attack - so they immediately un-stall without any input from the pilot. Of course if you lose the motor - the plane is going to nose down into the ground...but it won't stall - so you stand at least a chance of pulling out of the dive when you've built up enough speed.
- The other odd thing about not having pitch control is that you can't fly faster or slower...if you add power without altering your pitch - you'll climb - and if you reduce power without changing pitch - you'll lose altitude. At a particular pitch there is only exactly one speed that'll keep you flying level! I find it hard to believe that this contraption really doesn't have some means to control pitch - I imagine that what they REALLY mean is that the pilot has no direct control over pitch - it's hard to believe that the flight computer can't control pitch.
- SteveBaker (talk) 02:38, 25 March 2009 (UTC)
- I definitely agree with Steve here, the only reasonable explanation is that the pilot has no direct control over pitch. There must be a mechanism for pitch control for the airplane to be flyable. If there were no pitch control, a steady altitude could only be maintained at one speed, which would be a complex function of weight, center of gravity, and air density. It would even change during flight as fuel is consumed, or even if a passenger leans forward in their seat! In turbulent air natural stability would be the only way to maintain attitude (and therefore altitude). It's even possible that in turbulence near the ground, the aircraft could be put into an attitude where recovery with no pitch control is impossible. Finally, pitch control would be necessary to flare into the proper attitude for landing. So pitch control is basically required for any airplane, even if the pilot has no direct control. anonymous6494 03:43, 25 March 2009 (UTC)
- Some version of "fly by wire?" Making the car/plane "idiot-proof?" Edison (talk) 04:23, 25 March 2009 (UTC)
- After some more reading it seems the link you provided is for a powered parachute, which indeed has no pitch control. anonymous6494 06:40, 25 March 2009 (UTC)
Thanks so much for the solid feedback, and that's right- a powered parachute, although the term - the entire field actually - is new to me. There are clearly some pretty substantial differences between this vehicle and the Terrafugia Transition but I was assigned to write a feature about the SkyCar and was told it was a flying car plain and simple. Evidently that's not the case at all. Wolfgangus (talk) 06:57, 25 March 2009 (UTC)
The Parajet Sykcar doesn't look like it has no pitch control. It looks like a powered parachute, which I would think can be stalled given enough effort. I wonder if the statement was about the Moller Skycar M400, about which is said "the pilot's only inputs are speed and direction". It would certainly lack pitch control. DJ Clayworth (talk) 21:23, 25 March 2009 (UTC)
- The Moller contraption certainly has pitch control inside the computer system - but the pilot has very little to do with flying the machine at all. Mostly he enters a destination and lets the computer do all of the flying. But the Moller gets most of it's lift from the half dozen thusters - it's not particularly aerodynamic...and it doesn't really work. Beyond a few hover tests, it hasn't done much flying. They sold the prototype under the condition that whoever bought it would not allow it to be flown again. SteveBaker (talk) 22:54, 25 March 2009 (UTC)
Mount st Helens
[edit]hi,
i think i heard somewhere that when mt st helens erupted in 1980 that it released more corbon dioxide into the atmosphere than humans have done to date. Is this at all true or not?
thanks, --84.66.48.29 (talk) 10:37, 25 March 2009 (UTC)
- [1] while not discussing the 1980 eruption in particular helps put things in perspective Nil Einne (talk) 11:07, 25 March 2009 (UTC)
- Direct measurements of atmospheric carbon dioxide taken at the Mauna Loa Observatory say otherwise. Things to note at the linked page's image:
- The yearly natural oscillation is regular enough that it can be easily subtracted, leading to the red curve.
- The steady growth is non-negligible
- Pinatubo and Saint Hellen's eruptions don't show up at all.
- Dauto (talk) 14:43, 25 March 2009 (UTC)
- Direct measurements of atmospheric carbon dioxide taken at the Mauna Loa Observatory say otherwise. Things to note at the linked page's image:
I don't think volacno erruptions put out much CO2, but it does put out other gasses like SO2 which are far less nice. —Preceding unsigned comment added by 65.121.141.34 (talk) 14:46, 25 March 2009 (UTC)
- And the SO2 levels can be higher than those created by humans in the immediate surrounding area, but not when compared to all the sulfur dioxide created by humans worldwide. Also, volcanoes are an occasional thing, while human industry is relentless in adding pollution. So, you might want to evacuate the area around a volcano, during an eruption, because of all the pollution it puts out (among other reasons), but volcanoes are bit players on the world stage. There is, however, something quite rare called a supervolcano which is entirely different. StuRat (talk) 15:51, 25 March 2009 (UTC)
- I site I looked at said the less than 1% of CO2 came from volcanoes - compared with SO2, about 1/3 of which (17 million tonnes) comes from volcanoes (that is a rough estimate on several counts). - Jarry1250 (t, c) 21:08, 26 March 2009 (UTC)
- It's also going to vary dramatically from year to year. StuRat (talk) 08:06, 27 March 2009 (UTC)
Sunny Delight
[edit]Does anyone know what ingredient/chemical in Sunny Delight causes sterility? Thanks, Paper CB. Papercutbiology♫ (talk) (Sign here!) 11:28, 25 March 2009 (UTC)
- None of them. Our SunnyD article lists the ingredients. --Heron (talk) 12:12, 25 March 2009 (UTC)
- I heard a lecture on food that it was a dye...but I can't remember. Thanks though. My fears are relinquished. :) Papercutbiology♫ (talk) (Sign here!) 13:30, 25 March 2009 (UTC)
- Just keep the bottle cold or: "Benzene can form in soft drinks containing vitamin C, also called ascorbic acid, and either sodium benzoate or potassium benzoate." That is more likely to cause cancer than sterility (Even for high doses our article says "not known".) Soft drink companies are scrambling to reformulate their beverages, so check the label. This is one of those things where the hype is bigger than the known study result. Should have known we have an articleBenzene in soft drinks. - 76.97.245.5 (talk) 14:20, 25 March 2009 (UTC)
- Yellow 5 is often being incorrectly cited as lowering sperm count. Mountain Dew getting the brunt of the criticism. However, yellow 5 doesn't seem to be in SunnyD; maybe that's another common myth. -- 72.248.158.162 (talk) 14:38, 25 March 2009 (UTC)
- Thanks for all the answers. I did know we have an article on SunnyD, I just didn't find it helpful. Papercutbiology♫ (talk) (Sign here!) 15:25, 25 March 2009 (UTC)
Endocrine stress system
[edit]A chapter in an article I'm reading is called: Endocrine stress system. It says:
The basic components of the stress system include:
- the locus ceruleus/noradrenergic sympathetic system;
- the hypothalamic-pituitary-adrenal axis.
However, I wonder if the LC/NE sympathetic system is neural and not endocrine? Lova Falk (talk) 11:55, 25 March 2009 (UTC)
- Both. Our epinephrine article leads with "Epinephrine (also referred to as adrenaline; see Terminology) is a hormone and neurotransmitter." Further investigation will reveal to you that strong sympathetic neural stimulation will result in endocrine release of epinephrine and norepinephrine from the adrenal medulla. Cool, huh? --Scray (talk) 02:11, 26 March 2009 (UTC)
- Thank you, but I'm just getting more and more confused. In the article on norepinephrine it says: "As a stress hormone, norepinephrine affects parts of the brain where attention and responding actions are controlled." As far as I understand, a hormone is released into the blood stream. So norepinephrine is released into the bloodstream, the blood travels to the brain and the brain gets more attentive. Is that really correct? I thought that norepinephrine affected the brain as a neurotransmitter.
- The article also says: "It (norepinephrine) is released from the adrenal medulla into the blood as a hormone, and is also a neurotransmitter in the central nervous system and sympathetic nervous system where it is released from noradrenergic neurons." Isn't it more correct to say: "It is released from the adrenal medulla into the blood as a hormone, and it is released from the locus ceruleus as a neurotransmitter in the central nervous system and sympathetic nervous system." ??? Lova Falk (talk) 10:11, 26 March 2009 (UTC)
- Not sure if you're still watching this thread, but I suggest that you question your assumptions when things don't make sense. The first paragraph of our article on hormones states that hormones "are chemicals released by cells that affect cells in other parts of the body." and goes on to say that "Hormones in animals are often transported in the blood.". Likewise, the first paragraph of our article on neurotransmitters states that "Neurotransmitters are packaged into vesicles that cluster beneath the membrane on the presynaptic side of a synapse, and are released into the synaptic cleft, where they bind to receptors in the membrane on the postsynaptic side of the synapse." Thus, hormones act at a distance, and neurotransmitters act across a synaptic cleft (directly from one cell to the one on the other side of the cleft). If you go back and read what you'd quoted and what I'd said earlier in light of these facts, it's all consistent. --Scray (talk) 01:29, 30 March 2009 (UTC)
vegetable oil for fuel
[edit]pls i would want to know what properties of vegetable oils are compatible with that of fuel.i means what properties of vegetable oils makes it possible to be used for fuels —Preceding unsigned comment added by Peaceobioma (talk • contribs) 12:15, 25 March 2009 (UTC)
- Hydrocarbon and oil are good places to start. 76.97.245.5 (talk) 13:53, 25 March 2009 (UTC)
- You may also want to look at the articles biofuel and biodiesel (and the links therein). TenOfAllTrades(talk) 14:28, 25 March 2009 (UTC)
- Note that it's not economical to use new vegetable oils for fuel, as they cost far more than other fuels. However, waste vegetable oils, such as those collected from the fryers in fast food restaurants, can be economical, after filtering, but only for a small segment of the fuel industry, as waste vegetable oils aren't produced in the quantities needed to supply all our fuel needs. One side benefit, the exhaust smells yummy (although that could be a negative if it makes you always hungry). :-) StuRat (talk) 15:37, 25 March 2009 (UTC)
- The current price of Malaysian palm oil is about 500 USD per metric ton, which is comparable to a petrolum cost of about 65 USD per barrel. The current price of crude oil is a bit more than 50 USD per barrel ([2]). At those spot prices, a switch to biodiesel could be economical right now if supported by relatively minor government incentives.
- That said, palm oil experienced a temporary price spike last year (up to just over 800 USD per ton) due to a combination of drought and intense interest in biodiesel; it's price over the last few years has also been lower than 300 USD per ton. Meanwhile, the price of crude oil has also rollercoastered over the last few years, running as low as 20 USD at the start of this decade, and spiking above 145 USD per barrel last summer. Neither type of oil has been a poster child for price stability of late.
- So it's a bit of an overstatement to assert that non-waste vegetable oils are inherently uneconomic. While capacity to produce such oils certainly doesn't exist to replace all fuel uses of petroleum overnight, it is by no means a foregone conclusion that a gradual transition is impossible — nor is such a transition even unlikely. TenOfAllTrades(talk) 16:27, 25 March 2009 (UTC)
- Using foodstuffs as fuel is an inherently bad idea, as the recent US experiment with using corn to produce ethanol shows. The price of corn skyrocketed, making ethanol cost more than gasoline, even at it's peak prices. Meanwhile, food prices went up dramatically as a result, since growing corn became more profitable than other crops, due to government subsidies. Also, there's the argument that it's immoral to burn food to run cars when people are starving. Finally, there's the infrastructure problem. Refineries and gas stations could be modified to provide biodiesel from palm oil, but that would be an enormous expense which would only be justified if this approach would be economical in the long run, and there's no sign that it would be. That leaves people to buy palm oil on their own and produce biodiesel, which ends up being far more expensive, unless you start with waste oil. StuRat (talk) 17:21, 25 March 2009 (UTC)
- Using the wrong foodstuffs as fuel is an inherently bad idea, as is attempting to make the changeover too quickly. See ethanol fuel in Brazil for one case where it was done properly and successfully. The United States' example is simply a perfect demonstration of exactly the wrong way to manage the transition. Extracting ethanol from corn has a much lower energy output per cultivated acre of land – and indeed may be negative net output once the energy costs of harvesting, fermentation, and refining are factored in – compared to Brazil's sugar cane ethanol or tropical palm oil. The U.S. system of farm subsidies (for corn and other products) badly distorts the market, and probably encouraged even more farmers to make an ill-advised switch to corn. About the only thing the U.S. approach has going for it is that it has encouraged the development of infrastructure (from refineries and gas stations to individual flex-fuel motor vehicles) which can cope with ethanol and ethanol-blended fuel. That will pay off in spades if cellulosic ethanol (from switchgrass, most likely) or another technology matures sufficiently to provide large amounts of sustainable ethanol.
- Replacement of diesel with biodiesel would require no changes for end users or distributors; they're equivalent products for virtually all uses. The same supertankers that carry crude oil from Alaska and the Middle East can carry biodiesel or unrefined palm oil from the tropics. The same gas pumps which deliver diesel can pump biodiesel. The same city bus that belches diesel soot will run happily on biodiesel.
- Yes, different refining equipment would be required for biodiesel compared with conventional diesel, but that's not a problem. Refinery capacity can be built to keep pace with the supply of suitable oils — and minimal refining of edible oils is required compared to the refining required for most petroleum products. Biodiesel can be blended into the petroleum diesel supply chain at any point after the products are refined. If anything, you've missed the most significant infrastructure hurdle, which is that most private motor vehicles don't currently burn diesel. Still, that's a problem that can resolve itself over a period of many years, as the cost of biodiesel declines. TenOfAllTrades(talk) 18:37, 25 March 2009 (UTC)
- In the US, at least, most fuel is gasoline, so the goal would be to replace that. Isn't biodiesel thicker than gasoline ? That would make me think new pumps would be needed. They also need to add biodiesel to the selection switch or add separate dedicated pumps for it. Also, unless they intend to no longer carry regular gasoline (which sounds like a foolish idea in the short term), gas stations will need to install new tanks for biodiesel storage, unless they just happen to have a spare tank already installed. For those stations which already have regular diesel, they could switch those pumps over to biodiesel rather easily, but that's only a small portion of stations (most of which which cater to truckers) in the US. And building new refineries is highly problematic in the US, as nobody wants one near them. StuRat (talk) 22:56, 25 March 2009 (UTC)
- Picky, aren't you? Any filling station pump that can handle regular diesel can pump biodiesel. Any underground tank that is compatible with gasoline and diesel can quite comfortably accommodate biodiesel. If a facility already pumps diesel, there's no barrier to biodiesel at all — just start filling the tanks with biodiesel. Contrary to your claim that only a small portion of filling stations offer diesel, a 2005 study pegged the fraction at 42% in the United States: [3]. (That represents a sharp increase over the 30% which offered diesel in 2000; by now the fraction is probably over one half.)
- Most filling stations in the United States dispense multiple grades of gasoline (typically one 'regular' unleaded and one or two 'premium' higher-octane options). If demand existed, a gas station owner could choose to drop one of the premium grades of gasoline and dispense (bio)diesel instead. All of the in-ground plumbing would remain the same. (The U.S. had a similar experience twenty or thirty years ago with the phase-out of leaded gasoline, and much of that multiple-fuel infrastructure is still in place.) Pumping equipment is repaired and replaced on a regular schedule; a switchover from gasoline to diesel is relatively straightforward.
- Yes, most personal motor vehicles in the United States use gasoline — but that would gradually change if biodiesel offered a consistently lower-priced, carbon-neutral alternative. (Slow, steady growth of the biodiesel market will also ease the economic dislocations that could result from a more rapid shift in demand. If cellulosic ethanol turns out to work in the meantime, that's great — we don't have to have all the eggs in one basket.) The distribution infrastructure works equally well for diesel or gasoline, so it's a matter of raw ingredient supply and refining capacity. Build the refineries in the tropics if you can't get the NIMBYists to let you build them in the States—diesel is much safer to transport than refined gasoline. TenOfAllTrades(talk) 03:18, 26 March 2009 (UTC)
- The food vs fuel issue is a perpetual one and is not IMHO a simple matter. Firstly the causes of the 2007–2008 world food price crisis are in great dispute as the article testifies too. IMHO fuel demand was a factor but I personally doubt it was the primary factor rather it was a large combination of factors and blaming biofuels was a convient excuse for a large number of people with different purposes. Regardless though, as TOAT testifies, you can't lump all food crops together. For starters while palm oil is in widespread use, it's a controversial oil because of it's high saturated fat content. While there are obvious some cases when you would want that, in many other cases its use is controversial. Of course there is some demand from those who believe the health problems caused by saturated fats, particularly saturated fats from vegetable oils may be overrated (perhaps due to the influence of money from other oil producers who tend to be in the developed world on scientists) as well as from those who believe that when polyunsatured oils are used for cooking they produce transfats or free radicals in sufficient amounts that it is better to use saturated oils. Regardless though, whether palm oil will, or should have a role as a major food crop in the future is an area of much debate. More importantly, as the world food price crisis testifies to (for example, the price of rice and other food not used to make biofuels to any great extent were also greatly affect), just not using food crops doesn't actually help in itself. If the non food crops used to make biofuels takes over the land of food crops, as will happen if they fetch a better price, then that doesn't help it makes things worse. At least if you are using food crops you still have the food you just have to pay more for it. There is some suggestion that you can use things like jatropha which it is hoped will not compete for land with food crops but this remains an unproven suggestion. There's also the hope we will be able to use all the waste material from plants that currently goes to waste, but this will obviously include food crops such as palm oil. There's also the question of whether it's fair to refuse to use biofuels, if you aren't actually subsiding them solely for your desire to not raise food prices when effectively what your preventing is farmers (many in the developing world) from getting a fair market price for their goods as well as effectively discouraging people from farming. This gets into a whole raft of complicated issues like agricultural subisidies, globalisation, protectionism, food security and competiting interests in the developing world. Let's not forget that one of the reasons why corn was so cheap is because of US government subisidies and many people have argued the US government effectively outcompeted many developing countries leading to a great fall in their agricultural production which has had numerous ill effects. (The EU also of course has large subsidies and had been blamed for many of the same problems in different areas, e.g. sugar beet.) Of course one of the great concerns with palm oil is whether it's current development is sustainable, but again this gets in to a whole raft of complicated issues like whether it's fair to demand the countries in developing world keep their rainforests when they could be getting a better return by replacing them (particularly when many developed countries have cut down a significant portion of their natural forests), what length of time we're referring to as well as how any future Kyoto protocol will develop (I think there's great concern parts of it will be based on the level of forests at some set point of time which is not going to seem fair to those who have preserved their forests). Of course if we bring biofuel subsidies in to the mix, it gets a lot more complicated. N.B. I recall reading that Malaysia was planning to develop a processing plant for making biodiesel from pa
How many barrels in a tonne though? A quick search online suggests around 7 but not sure how reliable that is. 194.221.133.226 (talk) 16:46, 25 March 2009 (UTC)
- 'Around 7' is about right. The density of crude oil ranges from about 0.8 to 0.9 grams per cubic centimeter [4]. A barrel of oil is 42 US gallons or about 160 liters; the weight of a barrel is therefore 130 to 145 kilograms (roughly). At an even 1000 kilograms per metric ton, that's about seven barrels to the ton. TenOfAllTrades(talk) 18:39, 25 March 2009 (UTC)
- Note that comparing tonne to tonne or volume to volume is pointless in itself. We need to consider the energy density of the fuels. Our Energy density article gives a slightly higher energy density for crude oil compared to biodiesel but I'm not sure how palm oil which hasn't yet been processed in to biodiesel compares. On the other hand, palm oil can be used as a fuel with relatively little processing I believe Nil Einne (talk) 01:55, 26 March 2009 (UTC)
Red decoder screen reveals blue text?
[edit]Which exact shades of red and blue should somebody optimally use to make one of those decoder things? If I am not mistaken, it is often text or an image printed in blue ink, then hidden by red "noise". When you put a certain kind of red transparency over it, the red is filtered out and the text or image comes out clearly. Is there a certain shade of blue or red that work best for this? Pantone or otherwise? etc. Are the red screens usually made from a certain type of plastic or other material? --Sonjaaa (talk) 19:04, 25 March 2009 (UTC)
- You want to ideally have a color to match the red noise. When something is red transparent, it appears red because it is reflecting the red portion of the white light in the room back at you. Being transparent, appart from this it is letting all other light through. So when you hold it up to the decoder you want it to reflect the red noise and let the blue noise through, so a color most similar to the one of the noise you want to filter out is best.
- If you are trying to print your own, I'd make sure to use just ink from the red color cartrage and just ink from the blue color cartrage. What you want to avoid is having multiple frequencies of light mixed together. Generally a clear red plastic is easy and cheap to produce. Anythingapplied (talk) 20:56, 25 March 2009 (UTC)
- That's a very complicated - and entirely wrong - explanation! It's nothing to do with the film reflecting light. Red film allows red light to pass through it - and blocks all of the other colors. So when you look at (say) blue writing on a white background - the blue light is blocked - so the writing looks black - the white paper looks red because all of the other colors are filtered out. Hence you see black writing on a red background. The choice of color for the 'blue' should be something as far from 'red' as possible - so a sky blue would probably be the best choice - it is on the opposite side of the 'color wheel' - the complement to red. Inkjet printers have magenta, cyan, yellow and black inks - none of which is a particularly good match for the red filter. But printing a red/cyan picture should work pretty well. SteveBaker (talk) 23:33, 25 March 2009 (UTC)
- This reminds of the old home version of the television game show Jeopardy!, which used a red plastic sheet on the back of the game board to make the blue answers hidden behind red "noise" visible. It was used to keep the clues hidden when selecting agame sheet, but allow them to be seen once the sheet was put in place behind the game board. --Thomprod (talk) 16:38, 27 March 2009 (UTC)
Optical inversion, but not reversal?
[edit]I just bought a projecting alarm clock. It has a projector which can be aimed to project the time onto the bedroom wall or ceiling. There is a switch to select the colour of the projected time: red, blue, or green. The projector, from my (external) investigation, consists of red, blue, and green LED emitters behind an LCD shadow mask, and a simple, manually-focusable lens at the front. The shadow mask is inverted from ordinary pocket calculator or wristwatch LCD operation; the background is black, and the digits — made up of standard 7-segment-bar display grids (the type that display "88:88" when all segments are active) — are transparent. Thus, the LED light shining through the transparent digits creates the projected time display. Now here's the mystery: if I peer into the operating projector, the time display appears inverted, but not reversed. That is, if the time is 1:08, peering into the projector reveals 1:08, not 80:1. If the time is 12:03, peering into the projector reveals 15:03, not E0:51 (please use your imagination to make these digits out of straight line segments; a "1" has no base and no hook, but is just a straight line, an upside-down "2" looks like "5" and vice-versa, a reversed "3" looks like "E", and so on). I am probably overthinking this, but how is it that the time is projected correctly on the opposite wall when it is apparently flopped only in the vertical, not in the horizontal? —Scheinwerfermann T·C22:13, 25 March 2009 (UTC)
- Oops, I was underthinking: when I look at the projection on the opposite wall, I'm horizontally flopping myself. If I were to hold up a translucent screen in front of the projector and look in the direction of the projector, then the digits would appear horizontally flopped. I'm pretty sure that solves the mystery, yes? —Scheinwerfermann T·C23:50, 25 March 2009 (UTC)
Fault zone or short metamorphism
[edit]Is there another name for this that I am unaware of? Which form of metamorphism does this refer to? Thanks, Grsz11 22:49, 25 March 2009 (UTC)
- Changes in a fault zone during deformation are sometimes referred to as dynamic metamorphism. Is that what you meant? I've never heard of 'short metamorphism', what context was this in? Mikenorton (talk) 22:57, 25 March 2009 (UTC)
- Sorry, by short, I meant shock. Grsz11 23:51, 25 March 2009 (UTC)
- 'Shock' metamorphism is described as impact metamorphism in our article, a section that is a lot less than comprehensive (there's also impactite but that's only a stub). Basically it refers to the effects of very high strain-rate events such as you would get during a meteorite impact or a large volcanic explosion. This causes characteristic effects in some minerals, such as deformation lamellae (microfractures along which local melting has sometimes occurred) in quartz and locally wholesale melting of the rock, forming suevite [5], or local high slip-rate faulting causing melting of the fault walls forming pseudotachylite.Mikenorton (talk) 08:57, 26 March 2009 (UTC)
- This link [6] is to a page on shock metamorphism that looks pretty comprehensive. Mikenorton (talk) 10:14, 26 March 2009 (UTC)
- 'Shock' metamorphism is described as impact metamorphism in our article, a section that is a lot less than comprehensive (there's also impactite but that's only a stub). Basically it refers to the effects of very high strain-rate events such as you would get during a meteorite impact or a large volcanic explosion. This causes characteristic effects in some minerals, such as deformation lamellae (microfractures along which local melting has sometimes occurred) in quartz and locally wholesale melting of the rock, forming suevite [5], or local high slip-rate faulting causing melting of the fault walls forming pseudotachylite.Mikenorton (talk) 08:57, 26 March 2009 (UTC)
- We now have an article on Shock metamorphism. Mikenorton (talk) 18:13, 27 March 2009 (UTC)
- Sorry, by short, I meant shock. Grsz11 23:51, 25 March 2009 (UTC)
Earth Warming... not!
[edit]I've heard data saying that the Earth was cooling, not warming, despite global warming. If the Earth is showing symptoms of global warming, why do they say the planet is cooling?--24.4.54.96 (talk) 23:40, 25 March 2009 (UTC)
- In fact, the Earth is warming according to the Temperature record. --TeaDrinker (talk) 01:07, 26 March 2009 (UTC)
- It depends on your time scale, of course. We are considerably cooler than say the Jurassic Period, so if you used those two data points, you could say we are cooling off... Remember, it's all in how you organize your data... But, if you want to look in the recent past, say the last few hundred years, we are warming some... --Jayron32.talk.contribs 01:38, 26 March 2009 (UTC)
- Perhaps you should read the Global cooling article? I think the Global Cooling hypotheses recently were popular in the 1970s and 1980s, and there have been improvements in understanding of the climate, as well as better computer modeling simulations. -- JSBillings 02:04, 26 March 2009 (UTC)
- Also note that some areas may cool whilst others warm. - Akamad (talk) 02:23, 26 March 2009 (UTC)
- If the earth is cooling - it's doing it on geological time-scales. Our behavior is warming it up on human time-scales. So, it's possible that what we'll happen is heat the planet up by 10 degrees over the next 150 years - and half a million years later, global cooling will erase that gain. But it's too far off to really affect the answer. Although the world was warmer still at some times in the dim and distant past - and it might be cooler again in the distant future - but our problem is with NOW - the next generation of humans. We can clearly see a trend - it's upwards - and the consequences are serious indeed. It's not rocket science. Follow along with these calculations:
- According to Earth#Hydrosphere, our oceans contain 1.4x109km3 of water.
- According to Coefficient of thermal expansion, water expands in volume at a little over 200 parts per million for every degree centigrade that the temperature rises.
- So - when the temperature goes up an average of 1 degree centigrade across the globe, we gain 200x10-6x1.4x109 cubic kilometers of water. That's 280,000 cubic kilometers of water.... 280,000,000,000,000 cubic meters.
- According to Earth, our oceans cover about 360,000,000,000,000 square meters of the earth's surface.
- Which mean that all of those zeroes cancel out and our oceans get 280/360 = 0.78 meters deeper every time we warm up the planet by just one degree.
- According to Effects of global warming - we've already seen a 20cm rise in global ocean levels since the 1920's from a third of a degree global temperature rise. This fits perfectly with the numbers I've just calculated.
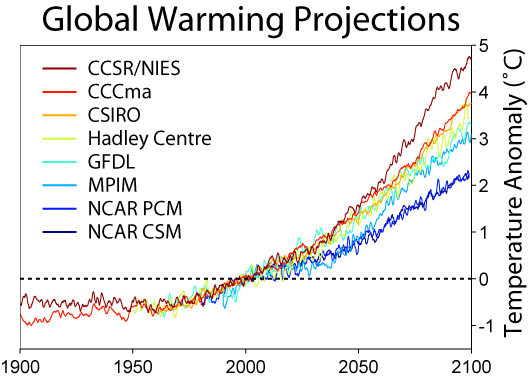
- Now - take a look at the graph to the right here. It shows the best estimates of temperature rise from the eight leading climatological institutes. They certainly disagree - but you've gotta admit we're going to see a degree or two of rise in our lifetimes - two to five degrees in our kid's lifetimes. Note that the graphs aren't levelling out - in most of them, the curve is getting steeper and steeper.
- So we should expect to see several meters of ocean level rise. Because most decent farmland is in low-lying river basins - we'll find that at least 150 million people will lose their livelyhood as a result of a ONE meter sea level rise. We're certainly going to get a lot more than that.
- But this is forgetting melting ice and all of that stuff. The melting of ice in Greenland alone is responsible for 260 cubic kilometers of water being dumped into the oceans every year...factoring that in - we're getting oceans that are going to be several meters deeper in the immediate future - and between 7 and 20 meters deeper in 100 years. Imagine yourself at your favorite seaside resort - now imagine the water at high tide being SEVENTY FEET deeper than it is right now. Do the same thing at any place with a big river flowing through it. London, NewYork, LA - completely gone.
- Denying what's happening right under our noses has gone beyond mere healthy skepticism. We're getting into the realms of obstructing the survival of modern civilisation.
- SteveBaker (talk) 03:26, 26 March 2009 (UTC)
- Did you just make that up about rivers being similarly affected? I would imagine that inland rivers would get lower as their snow & ice sources get smaller. --Sean 12:33, 26 March 2009 (UTC)
- Well, eventually...but I'm not talking about mountain streams here - I'm talking about the close-to-sea-level river deltas. These were ideal places to start major cities - and they are also big-time sources of agricultural land...and when the ocean levels rise - they vanish. SteveBaker (talk) 19:55, 26 March 2009 (UTC)
- "London, NewYork, LA" don't really have "inland" rivers. I'm going to go out on a limb and say that the rivers in those cities are probably very much affected by sea level. APL (talk) 12:46, 26 March 2009 (UTC)
- Did you just make that up about rivers being similarly affected? I would imagine that inland rivers would get lower as their snow & ice sources get smaller. --Sean 12:33, 26 March 2009 (UTC)
- At the risk of sounding like a denialist, do those temperature scales from 1900 to present account for the urban heat island effect? In other words, areas with thermometers that were rural in 1900 may now be urban today, and would gain heat as a result. 65.121.141.34 (talk) 13:25, 26 March 2009 (UTC
- I think those are average temperatures over the entire world. The urban heat island effect doesn't do much to average temperatures, just the ones in urban areas. --Tango (talk) 15:59, 26 March 2009 (UTC)
- I honestly think the climatologists would have thought of that! If just one of those institutes was claiming this kind of rise and the others were not - then we could wonder whether they had made such a major boo-boo. But this goes well beyond that. They ALL pretty much agree - and they aren't measuring this with thermometers hung out of their office windows...we're talking ice-cores in the antarctic, tree rings, satellite thermal imagery...this is a pretty seriously researched topic. SteveBaker (talk) 19:55, 26 March 2009 (UTC)
- I think those are average temperatures over the entire world. The urban heat island effect doesn't do much to average temperatures, just the ones in urban areas. --Tango (talk) 15:59, 26 March 2009 (UTC)
- At the risk of sounding like a denialist, do those temperature scales from 1900 to present account for the urban heat island effect? In other words, areas with thermometers that were rural in 1900 may now be urban today, and would gain heat as a result. 65.121.141.34 (talk) 13:25, 26 March 2009 (UTC
- One other note on the expansion of water part. The math is wrong in that it assumes that the oceans will warm evenly. In fact, only the top layer will warm significantly. At 10,000 ft deep the water will still be about freezing. 65.121.141.34 (talk) 13:27, 26 March 2009 (UTC)
- True. Most of the rise in sea levels is from melting polar ice. I think the accuracy of Steve's figures is a coincidence! --Tango (talk) 15:59, 26 March 2009 (UTC)
- But just the ice from Antarctica, correct? Most (though not all) of the arctic ice is floating, and Archimedes says that the water displaced by that ice must be equal to mass of the ice itself. Matt Deres (talk) 14:08, 29 March 2009 (UTC)
- I believe you're partially wrong [7] [8] [9] [10] and Ice shelf#Ice shelf disruption Nil Einne (talk) 14:20, 29 March 2009 (UTC)
- Damn facts getting in the way of things... Matt Deres (talk) 21:48, 29 March 2009 (UTC)
- I believe you're partially wrong [7] [8] [9] [10] and Ice shelf#Ice shelf disruption Nil Einne (talk) 14:20, 29 March 2009 (UTC)
- But just the ice from Antarctica, correct? Most (though not all) of the arctic ice is floating, and Archimedes says that the water displaced by that ice must be equal to mass of the ice itself. Matt Deres (talk) 14:08, 29 March 2009 (UTC)
- True. Most of the rise in sea levels is from melting polar ice. I think the accuracy of Steve's figures is a coincidence! --Tango (talk) 15:59, 26 March 2009 (UTC)
- One other note on the expansion of water part. The math is wrong in that it assumes that the oceans will warm evenly. In fact, only the top layer will warm significantly. At 10,000 ft deep the water will still be about freezing. 65.121.141.34 (talk) 13:27, 26 March 2009 (UTC)
- I believe those predictions are made assuming we continue pumping out CO2 at ever increasing rates as we've been doing previously. If we do cut back on emissions, we can significantly reduce (but certainly not eliminate) the risks. So far, that isn't happening, though - until China starts taking steps to reign in its increasing emissions, there isn't a great deal the rest of the world can do (every little helps, of course). --Tango (talk) 15:59, 26 March 2009 (UTC)
- That's an odd claim. China produces about a quarter of the world's CO2 emissions. That leaves plenty for the rest of the world to do without China onside. Algebraist 19:23, 26 March 2009 (UTC)
- It's not absolute amounts, it's increases/reductions. China is increasing its emissions at a rate that more than cancels out everyone else's reductions (I think - it's been a while since I examined the numbers). Also, China hasn't picked all the low hanging fruit that everyone else has done, so could make significant reductions if they tried (or, at least, significantly slow down their increases - they couldn't actually reduce without dramatically slowing their economic growth, which they won't do and it's a bit much for other countries to ask them to). --Tango (talk) 22:23, 26 March 2009 (UTC)
- That's an odd claim. China produces about a quarter of the world's CO2 emissions. That leaves plenty for the rest of the world to do without China onside. Algebraist 19:23, 26 March 2009 (UTC)
- No, but they are also accellerating their CO2 production far more quickly then Europe or even the US. 65.121.141.34 (talk) 20:13, 26 March 2009 (UTC)
- The fact is, if the US and Europe don't take the lead, nobody will. In any case, per person the US's CO2 production is an order of magnitude more than the Chinese. If the US isn't willing to cut back, why would China? In other areas of strategic importance (say, nuclear weapons stockpiles), when the US (which has a lot more) refuses to make reasonable and appropriate reductions, nobody else (like China, Russia, etc.) has ever felt the need to do it instead. When the US does make such reductions, it gives it moral and political leverage (and leverage with other nations, which can apply additional weight to it) when it makes requests of others. The "China isn't doing it, so why should we?" is a pretty silly argument—one that guarantees that no one will reduce if everyone follows it. --140.247.249.53 (talk) 22:03, 26 March 2009 (UTC)
- I never said others shouldn't do their bit anyway, I just said without China doing something it's not going to help much. --Tango (talk) 22:23, 26 March 2009 (UTC)
- Well I think that point is somewhat in dispute since 1) If every single other countries stop emitting all CO2 it would have a significant effect in itself for a long time. 2) Given 1, China's emissions would go down too anyway since they won't be buying all those goods coming out of China since they'd have massive populations crashes. Of course, 1 is not a sensible suggestion but it doesn't change the fact Nil Einne (talk) 14:25, 29 March 2009 (UTC)
- I never said others shouldn't do their bit anyway, I just said without China doing something it's not going to help much. --Tango (talk) 22:23, 26 March 2009 (UTC)
- The fact is, if the US and Europe don't take the lead, nobody will. In any case, per person the US's CO2 production is an order of magnitude more than the Chinese. If the US isn't willing to cut back, why would China? In other areas of strategic importance (say, nuclear weapons stockpiles), when the US (which has a lot more) refuses to make reasonable and appropriate reductions, nobody else (like China, Russia, etc.) has ever felt the need to do it instead. When the US does make such reductions, it gives it moral and political leverage (and leverage with other nations, which can apply additional weight to it) when it makes requests of others. The "China isn't doing it, so why should we?" is a pretty silly argument—one that guarantees that no one will reduce if everyone follows it. --140.247.249.53 (talk) 22:03, 26 March 2009 (UTC)
The calculation about the ocean water expanding if the global temperature rose makes the unsupported assumption that the water would have a constant quantity (or mass). Higher surface temperature would mean more evaporation, putting more water in the atmosphere. On the other hand, melting ice (at least the ice on land) would increase the mass of water in the ocean, but would slightly lower its density by diluting the salt. How would global precipitation be affected, and how would underground aquifers be affected, as people pump the water table down lower and lower? We have also seen the water level in lakes drop dramatically due to lower rain and higher usage in some regions. How about biomass: desert versus jungle/forest/cropland, where some water is bound up in plants and soil. There are several factors which would increase and several which would decrease the mass of water in the ocean. 19:09, 26 March 2009 (UTC)
- You're kidding right? You think that a 1 degree temperature rise will evaporate a quarter of a million cubic kilometers of water - and that won't have a drastic effect? Let me point out a little something...water vapor is a MUCH nastier greenhouse gas than CO2. If anything remotely close to that much water made it into the atmosphere - it would be approximately like living on the surface of venus! But it's silly - even at 100% humidity - there wouldn't be "room" in the atmosphere for a quarter of a million cubic kilometers of liquid-water-turned-into-vapor. SteveBaker (talk) 19:50, 26 March 2009 (UTC)
- The total water vapor content of the atmosphere amounts to about 3 cm of sea level equivalent. Dragons flight (talk) 22:14, 26 March 2009 (UTC)
Venus to earth atmosphere analogy is next to worthless because we are not going to get anywhere near 96.5% CO2. I believe right now we are at 0.035% CO2 give or take. And at levels above 8% you have 10 minutes to live anyways so temperature would not matter much. Plus there is a little thing called condensation and cloud formation, more prevalent with higher degrees of water vapor which would reflect more sunlight (a cooling effect I believe) right? 65.121.141.34 (talk) 20:10, 26 March 2009 (UTC)
- I've heard this claim used countless times by AGW skeptics, that the Earth has been cooling since 1998. It's because 1998 and 2005 were the top two warmest years on record, 2005 being the warmest with 1998 at a close second. Some say that 1998 was the warmest year, therefore global warming has stopped. That's not true. 1998 was in the midst of a strong El Nino followed quickly by another strong La Nina. Most of the years in the past decade have been among the top ten warmest years on record, but there is always variability in the level of warming across the globe. Also, most climate models do not factor in the effects of positive feedbacks, especially recent ones such as the release of methane clathrates. The IPCC keeps the maximum expected sea level rise this century under one metre, but it is based on information that is several years old. Also, there are likely to be areas where ice sheets such as West Antarctica could be destabilised from, such as Pine Island Bay, one of the fastest-warming areas in Antarctica outside of the Antarctic Peninsula. When sea level rise occurs around rivers, the ocean can flood farther inland upstream the river. Another possible scenario, if the sea level rise exceeds about 25 m, is that water could flow past Lake Manych-Gudilo. However, us having a climate like Venus is not very likely. ~AH1(TCU) 01:09, 28 March 2009 (UTC)
