Blue: Difference between revisions
→Plants and fungi: ref |
|||
| Line 174: | Line 174: | ||
===Plants and fungi=== |
===Plants and fungi=== |
||
Intense efforts have focused on blue flowers and the possibility that natural blue colorants could be used as food dyes.<ref>{{cite book |title=Anthocyanins: Biosynthesis, Functions, and Applications|editor=Chris Winefield and Kevin M Davies|publisher=Wiley-VCH|year=2009|isbn=9781489989550}}</ref><ref name=Ag/> |
Intense efforts have focused on blue flowers and the possibility that natural blue colorants could be used as food dyes.<ref>{{cite book |title=Anthocyanins: Biosynthesis, Functions, and Applications|editor=Chris Winefield and Kevin M Davies|publisher=Wiley-VCH|year=2009|isbn=9781489989550}}</ref><ref name=Ag/> Common blue colorants of plants are [[anthocyanin]]s.<ref>{{cite book |editor1-last=Gould |editor1-first=K. |editor2-last=Davies |editor2-first=K. |editor3-last=Winefield |editor3-first=C. |title=Anthocyanins: Biosynthesis, Functions, and Applications |publisher=Springer |date=2008 |isbn=978-0-387-77334-6 }}</ref> |
||
<gallery> |
<gallery> |
||
File:Blaue Primeln.JPG|''Primula acaulis'' |
File:Blaue Primeln.JPG|''Primula acaulis'' |
||
Revision as of 21:13, 23 June 2022
| Blue | |
|---|---|
| Spectral coordinates | |
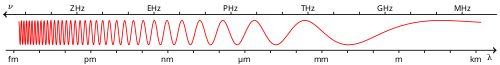
| Wavelength | approx. 450–495 nm |
| Frequency | ~670–610 THz |
| Hex triplet | #0000FF |
| sRGBB (r, g, b) | (0, 0, 255) |
| HSV (h, s, v) | (240°, 100%, 100%) |
| CIELChuv (L, C, h) | (32, 131, 266°) |
| Source | HTML/CSS[1] |
| B: Normalized to [0–255] (byte) H: Normalized to [0–100] (hundred) | |
Blue is one of the three primary colours in the RYB colour model (traditional color theory), as well as in the RGB (additive) colour model.[2] It lies between violet and cyan on the spectrum of visible light. The eye perceives blue when observing light with a dominant wavelength between approximately 450 and 495 nanometres. Most blues contain a slight mixture of other colours; azure contains some green, while ultramarine contains some violet. The clear daytime sky and the deep sea appear blue because of an optical effect known as Rayleigh scattering. An optical effect called Tyndall effect explains blue eyes. Distant objects appear more blue because of another optical effect called aerial perspective.
-
Pure blue, also known as high blue, is not mixed with any other colours
-
Egyptian blue goblet from Mesopotamia, 1500–1300 BC. This was the first synthetic blue, first made in about 2500 BC.
-
Extract of natural indigo, the most popular blue dye before the invention of synthetic indigo
-
A block of lapis lazuli, originally used to make ultramarine
-
Ultramarine, slightly violet-blue, in The Wilton Diptych (1395–1399). It was the most expensive pigment of Renaissance.
-
Medium blue, a shade of blue in between darker and lighter shades of blue.
Science and technology
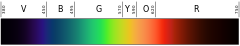
| Colour | Frequency (THz) |
Wavelength (nm) |
|---|---|---|
| 668–789 | 380–450 | |
blue
|
606–668 | 450–495 |
| 526–606 | 495–570 | |
| 508–526 | 570–590 | |
| 484–508 | 590–620 | |
| 400–484 | 620–770 | |
Human eyes perceive blue when observing light which has a dominant wavelength of roughly 450–495 nanometres. Blues with a higher frequency and thus a shorter wavelength gradually look more violet, while those with a lower frequency and a longer wavelength gradually appear more green. Pure blue, in the middle, has a wavelength of 470 nanometres.
Isaac Newton included blue as one of the seven colours in his first description the visible spectrum. He chose seven colours because that was the number of notes in the musical scale, which he believed was related to the optical spectrum. He included indigo, the hue between blue and violet, as one of the separate colours, though today it is usually considered a hue of blue.[3]
In painting and traditional colour theory, blue is one of the three primary colours of pigments (red, yellow, blue), which can be mixed to form a wide gamut of colours. Red and blue mixed together form violet, blue and yellow together form green. Mixing all three primary colours together produces a dark grey. From the Renaissance onward, painters used this system to create their colours. (See RYB colour model.)
The RYB model was used for colour printing by Jacob Christoph Le Blon as early as 1725. Later, printers discovered that more accurate colours could be created by using combinations of cyan, magenta, yellow, and black ink, put onto separate inked plates and then overlaid one at a time onto paper. This method could produce almost all the colours in the spectrum with reasonable accuracy.
-
Additive colour mixing. The projection of primary colour lights on a screen shows secondary colours where two overlap; the combination red, green, and blue each in full intensity makes white.
-
Blue and orange pixels on an LCD television screen. Closeup of the red, green and blue sub-pixels on left.
On the HSV colour wheel, the complement of blue is yellow; that is, a colour corresponding to an equal mixture of red and green light. On a colour wheel based on traditional colour theory (RYB) where blue was considered a primary colour, its complementary colour is considered to be orange (based on the Munsell colour wheel).[4]
Lasers emitting in the blue region of the spectrum became widely available to the public in 2010 with the release of inexpensive high-powered 445–447 nm laser diode technology.[5] Previously the blue wavelengths were accessible only through DPSS which are comparatively expensive and inefficient, but still widely used by scientists for applications including optogenetics, Raman spectroscopy, and particle image velocimetry, due to their superior beam quality.[6] Blue gas lasers are also still commonly used for holography, DNA sequencing, optical pumping, among other scientific and medical applications.
Shades and variations

Blue is the colour of light between violet and cyan on the visible spectrum. Hues of blue include indigo and ultramarine, closer to violet; pure blue, without any mixture of other colours; Cyan, which is midway in the spectrum between blue and green, and the other blue-greens such as turquoise, teal, and aquamarine.
Blue also varies in shade or tint; darker shades of blue contain black or grey, while lighter tints contain white. Darker shades of blue include ultramarine, cobalt blue, navy blue, and Prussian blue; while lighter tints include sky blue, azure, and Egyptian blue. (For a more complete list see the List of colours).
Blue as a structural color
-
The brilliant iridescent colors of the peacock's tail feathers are created by structural coloration, as first noted by Isaac Newton and Robert Hooke.
-
Indigo buntings as well as bluejays owe their blue color to structural effects, not blue pigments.[7]
In nature, many blue phenomena arise from structural coloration, the result of interference between reflections from two or more surfaces of thin films, combined with refraction as light enters and exits such films. The geometry then determines that at certain angles, the light reflected from both surfaces interferes constructively, while at other angles, the light interferes destructively. Diverse colours therefore appear despite the absence of colorants.[8] In animals such as on the feathers of birds and the scales of butterflies, interference is created by a range of photonic mechanisms, including diffraction gratings, selective mirrors, photonic crystals, crystal fibres, matrices of nanochannels and proteins that can vary their configuration. Some cuts of meat also show structural coloration due to the exposure of the periodic arrangement of the muscular fibres. Many of these photonic mechanisms correspond to elaborate structures, which are visible by electron microscopy. In the few plants that exploit structural coloration, brilliant colours are produced by structures within cells. The most brilliant blue coloration known in any living tissue is found in the marble berries of Pollia condensata, where a spiral structure of cellulose fibrils scattering blue light. Other examples include bird feathers and iridescent butterfly wings. The fruit of quandong (Santalum acuminatum) can appear blue owing to the same effect.[9]
Blue colorants
-
Egyptian blue, the first artificial pigment, produced in the third millennium BC in Ancient Egypt by grinding sand, copper and natron, and then heating them. It was often used in tomb paintings and funereal objects to protect the dead in their afterlife.
-
First produced in the 1930s, the intensely blue copper phthalocyanine is widely used for making blue ink, dye, and pigment.
-
YInMn blue, an inorganic compound of yttrium, indium, and manganese, was discovered by Mas Subramanian and Andrew E. Smith in 2009.
History of blue colorants
Prior to the 1700's, blue colorants for artwork were based on lapis lazuli and the related mineral ultramarine. A major breakthrough occurred in1709 when German druggist and pigment maker Johann Jacob Diesbach discovered Prussian blue. The new blue arose from experiments involving heating dried blood with iron sulphides and was initially called Berliner Blau. By 1710 it was being used by the French painter Antoine Watteau, and later his successor Nicolas Lancret. It became immensely popular for the manufacture of wallpaper, and in the 19th century was widely used by French impressionist painters.[10] Beginning in the 1820s, Prussian blue was imported into Japan through the port of Nagasaki. It was called bero-ai, or Berlin blue, and it became popular because it did not fade like traditional Japanese blue pigment, ai-gami, made from the dayflower. Prussian blue was used by both Hokusai, in his famous wave paintings, and Hiroshige.[11]
In 1799 a French chemist, Louis Jacques Thénard, made a synthetic cobalt blue pigment which became immensely popular with painters.
In 1824 the Societé pour l'Encouragement d'Industrie in France offered a prize for the invention of an artificial ultramarine which could rival the natural colour made from lapis lazuli. The prize was won in 1826 by a chemist named Jean Baptiste Guimet, but he refused to reveal the formula of his colour. In 1828, another scientist, Christian Gmelin then a professor of chemistry in Tübingen, found the process and published his formula. This was the beginning of new industry to manufacture artificial ultramarine, which eventually almost completely replaced the natural product.[12]
In 1878 German chemists synthesized indigo. This product rapidly replaced natural indigo, wiping out vast farms growing indigo. It is now the blue of blue jeans. As the pace of organic chemistry accelerated, a succession of synthetic blue dyes were discovered including Indanthrone blue, which had even greater resistance to fading during washing or in the sun, and copper phthalocyanine.
-
Thomas Gainsborough's The Blue Boy includes "the lavish lapis lazuli, the darker indigo pigment and the paler cobalt."[13]
-
The 19th-century Japanese woodblock artist Hokusai used Prussian blue, a synthetic colour imported from Europe, in his wave paintings, such as in The Great Wave off Kanagawa.
-
A synthetic indigo dye factory in Germany in 1890. The manufacture of this dye ended the trade in indigo from America and India that had begun in the 15th century.
Dyes for textiles and food

Blue dyes are organic compounds, both synthetic and natural.[9] Woad and true indigo were once used but since the early 1900's, all indigo is synthetic. Produced on an industrial scale, indigo is the blue of blue jeans.
For food, the triarylmethane dye Brilliant blue FCF is used for candies. The search continues for stable, natural blue dyes suitable for the food industry.[9]
Pigments for painting and glass
Blue pigments were once produced from minerals, especially lapis lazuli and its close relative ultramarine. These minerals were crushed, ground into powder, and then mixed with a quick-drying binding agent, such as egg yolk (tempera painting); or with a slow-drying oil, such as linseed oil, for oil painting. Two inorganic but synthetic blue pigments are cerulean blue (primarily cobalt(II) stanate: Co
2SnO
4) and Prussian blue (milori blue: primarily Fe
7(CN)
18). The chromophore in blue glass and glazes is cobalt(II). Diverse cobalt(II) salts such as cobalt carbonate or cobalt(II) aluminate are mixed with the silica prior to firing. The cobalt occupies sites otherwise filled with silicon.
Inks
Methyl blue is the dominant blue pigment in inks used in pens.[14] Blueprinting involves the production of Prussian blue in situ.
Blue inorganic ions
-
CuSO4.5H2O
-
Anhydrous cobalt(II) chloride
Certain metal ions characteristically form blue solutions or blue salts. Of some practical importance, cobalt is used to make the deep blue glazes and glasses. It substitutes for Si or Al ions in these materials. Cobalt is the blue chromophore in stained glass windows, such as those in Gothic cathedrals and in Chinese porcelain beginning in the T'ang Dynasty. Copper(II) (Cu2+) also gives many blue compounds including the commercial algicide copper sulfate (CuSO4.5H2O). Similarly, vanadyl salts and solutions are often blue, e.g. vanadyl sulfate.
Blue in nature
Sky and sea
When sunlight passes through the atmosphere, the blue wavelengths are scattered more widely by the oxygen and nitrogen molecules, and more blue comes to our eyes. This effect is called Rayleigh scattering, after Lord Rayleigh and confirmed by Albert Einstein in 1911.[15][16]
The sea is seen as blue for largely the same reason: the water absorbs the longer wavelengths of red and reflects and scatters the blue, which comes to the eye of the viewer. The colour of the sea is also affected by the colour of the sky, reflected by particles in the water; and by algae and plant life in the water, which can make it look green; or by sediment, which can make it look brown.[17]
Atmospheric perspective
The farther away an object is, the more blue it often appears to the eye. For example, mountains in the distance often appear blue. This is the effect of atmospheric perspective; the farther an object is away from the viewer, the less contrast there is between the object and its background colour, which is usually blue. In a painting where different parts of the composition are blue, green and red, the blue will appear to be more distant, and the red closer to the viewer. The cooler a colour is, the more distant it seems.[18]
-
Blue light is scattered more than other wavelengths by the gases in the atmosphere, giving the Earth a blue halo when seen from space.
-
An example of aerial, or atmospheric perspective. Objects become more blue and lighter in colour the farther they are from the viewer, because of Rayleigh scattering.
-
Under the sea, red and other light with longer wavelengths is absorbed, so white objects appear blue. The deeper the observer goes, the darker the blue becomes. In the open sea, only about one per cent of light penetrates to a depth of 200 metres. (See underwater and euphotic depth)
Astronomy
Blue giants are hot and luminous stars with surface temperatures exceeding 10,000 K. The largest blue supergiant stars are extremely massive and energetic, and are usually unstable. They are generally short-lived, either exploding in a supernova or periodically shedding their outer layers to become red giants.
Minerals
Some of the most desirable gems are blue, including sapphire and tanzanite. Compounds of copper(II) are characteristically blue and so are many copper-containing minerals.
Azurite (Cu
3(CO
3)
2(OH)
2), with a deep blue color, was once employed in medieval years, but it is unstable pigment, losing its color especially under dry conditions. Lapis lazuli, mined in Afghanistan for more than three thousand years, was used for jewelry and ornaments, and later was crushed and powdered and used as a pigment. The more it was ground, the lighter the blue colour became. Natural ultramarine, made was the finest available blue pigment in the Middle Ages and the Renaissance. It was extremely expensive, and in Italian Renaissance art, it was often reserved for the robes of the Virgin Mary.
Plants and fungi
Intense efforts have focused on blue flowers and the possibility that natural blue colorants could be used as food dyes.[19][9] Common blue colorants of plants are anthocyanins.[20]
-
Primula acaulis
-
Blue Delphinium flower
Animals
-
Blue facial ridges of mandrill
Some butterflies, such as Nessaea, have blue pigment, specifically pterobilin.[22] Some fish and frogs also have intensely blue chromatophores in their skin.
Eyes

Blue eyes do not actually contain any blue pigment. Eye colour is determined by two factors: the pigmentation of the eye's iris[23][24] and the scattering of light by the turbid medium in the stroma of the iris.[25] In humans, the pigmentation of the iris varies from light brown to black. The appearance of blue, green, and hazel eyes results from the Tyndall scattering of light in the stroma, an optical effect similar to what accounts for the blueness of the sky.[25][26] The irises of the eyes of people with blue eyes contain less dark melanin than those of people with brown eyes, which means that they absorb less short-wavelength blue light, which is instead reflected out to the viewer. Eye colour also varies depending on the lighting conditions, especially for lighter-coloured eyes.
Blue eyes are most common in Ireland, the Baltic Sea area and Northern Europe,[27] and are also found in Eastern, Central, and Southern Europe. Blue eyes are also found in parts of Western Asia, most notably in Afghanistan, Syria, Iraq, and Iran.[28] In Estonia, 99% of people have blue eyes.[29][30] In Denmark 30 years ago, only 8% of the population had brown eyes, though through immigration, today that number is about 11%.[30] In Germany, about 75% have blue eyes.[30]
In the United States, as of 2006, one out of every six people, or 16.6% of the total population, and 22.3% of the white population, have blue eyes, compared with about half of Americans born in 1900, and a third of Americans born in 1950. Blue eyes are becoming less common among American children. In the US, boys are 3–5 per cent more likely to have blue eyes than girls.[27]
See also
References
Notes and citations
- ^ "CSS Color Module Level 3". w3.org. Archived from the original on 2010-12-23.
- ^ Defonseka, Chris (2019-05-20). Polymeric Composites with Rice Hulls: An Introduction. Walter de Gruyter GmbH & Co KG. ISBN 978-3-11-064320-6.
- ^ Arthur C. Hardy and Fred H. Perrin. The Principles of Optics. McGraw-Hill Book Co., Inc., New York. 1932.
- ^ "Color wheel". Sanford-artedventures.com. Glossary Term.
{{cite web}}: CS1 maint: url-status (link) - ^ "Laserglow – Blue, Red, Yellow, Green Lasers". Laserglow.com. Archived from the original on 2011-09-16. Retrieved 2011-09-20.
- ^ "Laserglow – Optogenetics". Laserglow.com. Archived from the original on 2011-09-15. Retrieved 2011-09-20.
- ^ "How Birds Make Colorful Feathers".
- ^ "Iridescence in Lepidoptera". Natural Photonics (originally in Physics Review Magazine). University of Exeter. September 1998. Archived from the original on April 7, 2014. Retrieved April 27, 2012.
- ^ a b c d Newsome, Andrew G.; Culver, Catherine A.; Van Breemen, Richard B. (2014). "Nature's Palette: The Search for Natural Blue Colorants". Journal of Agricultural and Food Chemistry. 62 (28): 6498–6511. doi:10.1021/jf501419q. PMID 24930897.
- ^ Michel Pastoureau, Bleu – HIstoire d'une couleur, pp. 114–16
- ^ oger Keyes, Japanese Woodblock Prints: A Catalogue of the Mary A. Ainsworth Collection, R, Allen Memorial Art Museum, Oberlin College, 1984, p. 42, plate #140, p. 91 and catalogue entry #439, p. 185. for more on the story of Prussian blue in Japanese prints, see also the website of the Victoria and Albert Museum, London.
- ^ Maerz and Paul (1930). A Dictionary of Color New York: McGraw Hill p. 206
- ^ "Eight blue moments in art history". The Tate. Archived from the original on 2018-10-16. Retrieved 2018-10-16.
- ^ Placke, Mina; Fischer, Norbert; Colditz, Michael; Kunkel, Ernst; Bohne, Karl-Heinz (2016). "Drawing and Writing Materials". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–12. doi:10.1002/14356007.a09_037.pub2. ISBN 9783527306732.
- ^ "Why is the sky Blue?". ucr.edu. Archived from the original on 2015-11-02.
- ^ Near sunrise and sunset, most of the light we see comes in nearly tangent to the Earth's surface, so that the light's path through the atmosphere is so long that much of the blue and even green light is scattered out, leaving the sun rays and the clouds it illuminates red. Therefore, when looking at the sunset and sunrise, the colour red is more perceptible than any of the other colours."Human color vision and the unsaturated blue color of the daytime sky Archived 2011-07-15 at the Wayback Machine", Glenn S. Smith, American Journal of Physics, Volume 73, Issue 7, pp. 590–597 (2005).
- ^ Anne Marie Helmenstine. "Why Is the Ocean Blue?". About.com Education. Archived from the original on 2012-11-18.
- ^ Eva Heller, Psychologie de la couleur - Psychology of colour (2009), p. 14 (French translation).
- ^ Chris Winefield and Kevin M Davies, ed. (2009). Anthocyanins: Biosynthesis, Functions, and Applications. Wiley-VCH. ISBN 9781489989550.
- ^ Gould, K.; Davies, K.; Winefield, C., eds. (2008). Anthocyanins: Biosynthesis, Functions, and Applications. Springer. ISBN 978-0-387-77334-6.
- ^ Harmon, A. D.; Weisgraber, K. H.; Weiss, U. (1980). "Preformed azulene pigments of Lactarius indigo (Schw.) Fries (Russulaceae, Basidiomycetes)". Experientia. 36: 54–56. doi:10.1007/BF02003967. S2CID 21207966.
- ^ Vane-Wright, Richard I. (22 February 1979). "The coloration, identification and phylogeny of Nessaea butterflies (Lepidoptera : Nymphalidae)" (PDF). Bulletin of the British Museum (Natural History). Entomology Series. 38 (2): 27–56. Retrieved 8 February 2018.
- ^ Wielgus AR, Sarna T (2005). "Melanin in human irides of different color and age of donors". Pigment Cell Res. 18 (6): 454–64. doi:10.1111/j.1600-0749.2005.00268.x. PMID 16280011.
- ^ Prota G, Hu DN, Vincensi MR, McCormick SA, Napolitano A (1998). "Characterization of melanins in human irides and cultured uveal melanocytes from eyes of different colors". Exp. Eye Res. 67 (3): 293–99. doi:10.1006/exer.1998.0518. PMID 9778410.
- ^ a b Fox, Denis Llewellyn (1979). Biochromy: Natural Coloration of Living Things. University of California Press. p. 9. ISBN 978-0-520-03699-4. Archived from the original on 2015-10-03.
- ^ Mason, Clyde W. (1924). "Blue Eyes". Journal of Physical Chemistry. 28 (5): 498–501. doi:10.1021/j150239a007.
- ^ a b Douglas Belkin (October 17, 2006). "Don't it make my blue eyes brown Americans are seeing a dramatic color change". The Boston Globe. Archived from the original on February 23, 2012.
- ^ "Pigmentation, the Pilous System, and Morphology of the Soft Parts". altervista.org. Archived from the original on 26 July 2011.
- ^ statement by Hans Eiberg from the Department of Cellular and Molecular Medicine at the University of Copenhagen
- ^ a b c Weise, Elizabeth. (2008-02-05) More than meets the blue eye: You may all be related Archived 2012-09-10 at the Wayback Machine. Usatoday.com. Retrieved on 2011-12-23.
Bibliography
- Ball, Philip (2001). Bright Earth, Art, and the Invention of Colour. London: Penguin Group. p. 507. ISBN 978-2-7541-0503-3. (page numbers refer to the French translation).
- Gage, John (1993). Colour and Culture: Practice and Meaning from Antiquity to Abstraction. London and Paris: Thames and Hudson. ISBN 978-2-87811-295-5.
- Pastoureau, Michel (2000). Bleu: Histoire d'une couleur (in French). Paris: Editions du Seuil. ISBN 978-2-02-086991-1.
- Pastoureau, Michel (2010). Les couleurs de nos souvenirs (in French). Paris: Editions du Seuil. ISBN 978-2-02-096687-0.
- Balfour-Paul, Jenny (1998). Indigo. London: British Museum Press. ISBN 978-0-7141-1776-8.
- Varichon, Anne (2005). Couleurs: pigments et teintures dans les mains des peuples (in French). Paris: Editions du Seuil. ISBN 978-2-02-084697-4.
- Heller, Eva (2009). Psychologie de la couleur: effets et symboliques (in French). Munich: Pyramyd. ISBN 978-2-35017-156-2.
- Mollo, John (1991). Uniforms of The American Revolution in Color. Illustrated by Malcolm McGregor. New York: Stirling Publications. ISBN 978-0-8069-8240-3.
- Broecke, Lara (2015). Cennino Cennini's Il Libro dell'Arte: a New English Translation and Commentary with Italian Transcription. Archetype. ISBN 978-1-909492-28-8.
External links
 The dictionary definition of blue at Wiktionary
The dictionary definition of blue at Wiktionary Media related to blue at Wikimedia Commons
Media related to blue at Wikimedia Commons- "Friday essay: from the Great Wave to Starry Night, how a blue pigment changed the world", By Dr Hugh Davies, theconversation.com
















![Indigo buntings as well as bluejays owe their blue color to structural effects, not blue pigments.[7]](http://upload.wikimedia.org/wikipedia/commons/thumb/6/62/Indigo_Bunting_by_Dan_Pancamo_4.jpg/120px-Indigo_Bunting_by_Dan_Pancamo_4.jpg)




![Thomas Gainsborough's The Blue Boy includes "the lavish lapis lazuli, the darker indigo pigment and the paler cobalt."[13]](http://upload.wikimedia.org/wikipedia/commons/thumb/b/b6/The_Blue_Boy.jpg/203px-The_Blue_Boy.jpg)















![Lactarius indigo contains an azulene derivative, which are characteristically blue[21]]]](http://upload.wikimedia.org/wikipedia/commons/thumb/8/86/Lactarius_indigo_48568_edit.jpg/120px-Lactarius_indigo_48568_edit.jpg)