Frontotemporal dementia
| Frontotemporal dementia | |
|---|---|
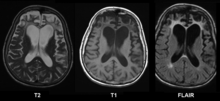 | |
| Brain MRI of a 65-year-old woman with frontotemporal dementia. Cortical and white matter atrophy of the frontal lobes is clear in all images. | |
| Specialty | Psychiatry, neurology |
| Causes | frontotemporal lobar degeneration |
Frontotemporal dementia (FTD), frontotemporal degeneration disease,[1] or frontotemporal neurocognitive disorder[2] encompasses several types of dementia involving the progressive degeneration of the brain's frontal and temporal lobes.[3] FTDs broadly present as behavioral or language disorders with gradual onsets.[4]
Common signs and symptoms include significant changes in social and personal behavior, disinhibition, apathy, blunting and dysregulation of emotions, and deficits in both expressive and receptive language.[5] Signs and symptoms tend to appear in late adulthood, typically between the ages of 45 and 65, although it can affect people younger or older than this.[1] Men and women appear to be equally affected.[1] FTD is the second most prevalent type of early onset dementia after Alzheimer's disease. Currently, there is no cure and no approved treatments to alleviate symptoms, although some off-label drugs and behavioral methods are prescribed.[1]
Each FTD subtype is relatively rare.[6] FTDs are mostly early onset syndromes linked to frontotemporal lobar degeneration (FTLD),[7] which is characterized by progressive neuronal loss predominantly involving the frontal or temporal lobes, and a typical loss of more than 70% of spindle neurons, while other neuron types remain intact.[8] The three main subtypes or variant syndromes are a behavioral variant (bvFTD) previously known as Pick's disease, and two variants of primary progressive aphasia (PPA): semantic (svPPA) and nonfluent (nfvPPA). Two rare distinct subtypes of FTD are neuronal intermediate filament inclusion disease (NIFID) and basophilic inclusion body disease (BIBD). Other related disorders include corticobasal syndrome (CBS or CBD), and FTD with amyotrophic lateral sclerosis (ALS).
Features of FTD were first described by Arnold Pick between 1892 and 1906.[9] The name Pick's disease was coined in 1922.[10] This term is now reserved only for the behavioral variant of FTD which shows the presence of the characteristic Pick bodies and Pick cells[11][12] first described by Alois Alzheimer in 1911.[10]
Signs and symptoms[edit]
Frontotemporal dementia is an early onset disorder that mostly occurs between the ages of 45 and 65,[13] but can begin earlier, and in 20–25% of cases onset is later.[7][14] Men and women appear to be equally affected.[15] It is the most common early presenting dementia.[16] FTD is the second most prevalent type of early onset dementia after Alzheimer's disease.[15][6]
The International Classification of Diseases recognizes the disease as causative to disorder affecting mental and behavioural aspects of the human organism. Dissociation from family, compulsive buying disorder (oniomania), vulgar speech characteristics, screaming, inability to control emotions, behavior, personality, and temperament are characteristic social display patterns.[17] A gradual onset and progression of changes in behavior or language deficits are reported to have begun several years prior to presentation to a neurologist.[15]
[edit]
The main subtypes of frontotemporal dementia are behavioral variant FTD (bvFTD), two variants of primary progressive aphasia – semantic dementia (svPPA) and progressive nonfluent aphasia (nfvPPA)[4][18] – as well as FTD associated with amyotrophic lateral sclerosis (FTD–ALS or FTD-MND).[15] Two distinct rare subtypes are neuronal intermediate filament inclusion disease (NIFID), and basophilic inclusion body disease (BIBD). Related disorders are corticobasal syndrome (CBS or CBD), and progressive supranuclear palsy (PSP).[15]
Behavioral variant frontotemporal dementia[edit]
Behavioral variant frontotemporal dementia (BvFTD) was previously known as Pick's disease, and is the most common of the FTD types.[19][18] BvFTD is diagnosed four times as often as the PPA variants.[20] Behavior can change in BvFTD in either of two ways—it can change to being impulsive and disinhibited, acting in socially unacceptable ways; or it can change to being listless and apathetic.[21][22] About 12–13% of people with bvFTD develop motor neuron disease.[23]
The Pick bodies in behavioral variant FTD are spherical inclusion bodies found in the cytoplasm of affected cells. They consist of tau fibrils as a major component together with a number of other protein products including ubiquitin and tubulin.[24]
Semantic dementia[edit]
Semantic dementia (SD) is characterized by the loss of semantic understanding, resulting in impaired word comprehension. However, speech remains fluent and grammatical.[22]
Progressive nonfluent aphasia[edit]
Progressive nonfluent aphasia (PNFA) is characterized by progressive difficulties in speech production.[22]
Neuronal intermediate filament inclusion disease[edit]
Neuronal intermediate filament inclusion disease (NIFID) is a rare distinct variant. The inclusion bodies that are present in NIFID are cytoplasmic and made up of type IV intermediate filaments.[25][26] NIFID has an early age of onset between 23 and 56. Symptoms can include behavioural, and personality changes, memory and cognitive impairments, language difficulties, motor weakness, and extrapyramidal symptoms.[25] NIFID is one of the frontotemporal lobar degeneration (FTLD)-FUS proteopathies.[26] Imaging commonly shows atrophy in the frontotemporal region, and in part of the striatum in the basal ganglia. Post-mortem studies show a marked reduction in the caudate nucleus of the striatum; frontotemporal gyri are narrowed, with widened intervening sulci, and the lateral ventricles are enlarged.[25]
Basophilic inclusion body disease[edit]
Another rare FTD variant, also a FTLD-FUS proteopathy, is basophilic inclusion body disease (BIBD).[27][28]
Other characteristics[edit]
In later stages of FTD, the clinical phenotypes may overlap.[22] People with FTD tend to struggle with binge eating and compulsive behaviors.[29] Binge eating habits are often associated with changes in food preferences (cravings for more sweets, carbohydrates), eating inedible objects and snatching food from others. Recent findings from structural MRI research have indicated that eating changes in FTD are associated with atrophy (wasting) in the right ventral insula, striatum, and orbitofrontal cortex.[29]
People with FTD show marked deficiencies in executive functioning and working memory.[30] Most become unable to perform skills that require complex planning or sequencing.[31] In addition to the characteristic cognitive dysfunction, a number of primitive reflexes known as frontal release signs are often able to be elicited. Usually the first of these frontal release signs to appear is the palmomental reflex which appears relatively early in the disease course whereas the palmar grasp reflex and rooting reflex appear late in the disease course.[citation needed]
In rare cases, FTD can occur in people with amyotrophic lateral sclerosis (ALS), a motor neuron disease. As of 2005[update], the prognosis for people with ALS was worse when combined with FTD, shortening survival by about a year.[32]
Genetics[edit]
A higher proportion of frontotemporal dementias seem to have a familial component than other neurodegenerative diseases such as Alzheimer's disease. More and more mutations and genetic variants are being identified all the time, needing constant updating of genetic influences.
- Tau-positive frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) is caused by mutations in the MAPT gene on chromosome 17 that encodes the tau protein.[33] It has been determined that there is a direct relationship between the type of tau mutation and the neuropathology of gene mutations. The mutations at the splice junction of exon 10 of tau lead to the selective deposition of the repetitive tau in neurons and glia. The pathological phenotype associated with mutations elsewhere in tau is less predictable, with both typical neurofibrillary tangles (consisting of both 3-repeat and 4-repeat tau) and Pick bodies (consisting of 3-repeat tau) having been described. The presence of tau deposits within glia is also variable in families with mutations outside of exon 10. This disease is now informally designated FTDP-17T. FTD shows a linkage to the region of the tau locus on chromosome 17, but it is believed that there are two loci leading to FTD within megabases of each other on chromosome 17.[34] The only other known autosomal dominant genetic cause of FTLD-tau is a hypomorphic mutation in VCP which is associated with a unique neuropathology called vacuolar tauopathy.[35]
- FTD caused by FTLD-TDP43 has numerous genetic causes. Some cases are due to mutations in the GRN gene, also located on chromosome 17. Others are caused by hypomorphic VCP mutations, although these patients present with a complex picture of multisystem proteinopathy that can include amyotrophic lateral sclerosis, inclusion body myopathy, Paget's disease of bone, and FTD. The most recent addition to the list (as of 2019[update]) was a hexanucleotide repeat expansion in intron 1 of C9ORF72.[36][37][38] Only one or two cases have been reported describing TARDBP (the TDP-43 gene) mutations in a clinically pure FTD (FTD without ALS).[citation needed]
- Several other genes have been linked to this condition. These include CYLD, OPTN, SQSTM1 and TBK1.[39] These genes have been implicated in the autophagy pathway.
- No genetic causes of FUS pathology in FTD have yet been reported.[citation needed]
- Major alleles of TMEM106B SNPs have been found to be associated with risk of FTLD.[40]
Pathology[edit]
There are three main histological subtypes found at post-mortem: FTLD-tau, FTLD-TDP, and FTLD-FUS. In rare cases, patients with clinical FTD were found to have changes consistent with Alzheimer's disease on autopsy.[41] The most severe brain atrophy appears to be associated with behavioral variant FTD, and corticobasal degeneration.[42]
With regard to the genetic defects that have been found, repeat expansion in the C9orf72 gene is considered a major contribution to FTLD, although defects in the GRN and MAPT genes are also associated with it.[43]
DNA damage and the defective repair of such damages have been etiologically linked to various neurodegenerative diseases including FTD.[44]
Diagnosis[edit]
FTD is traditionally difficult to diagnose owing to the diverse nature of the associated symptoms. Signs and symptoms are classified into three groups based on the affected functions of the frontal and temporal lobes:[12] These are behavioural variant frontotemporal dementia, semantic dementia, and progressive nonfluent aphasia. An overlap between symptoms can occur as the disease progresses and spreads through the brain regions.[14]
Structural MRI scans often reveal frontal lobe and/or anterior temporal lobe atrophy, but in early cases the scan may seem normal. Atrophy can be either bilateral or asymmetric.[13] Registration of images at different points of time (e.g., one year apart) can show evidence of atrophy that otherwise at individual time points may be reported as normal. Many research groups have begun using techniques such as magnetic resonance spectroscopy, functional imaging, and cortical thickness measurements in an attempt to offer an earlier diagnosis to the FTD patient. Fluorine-18-fluorodeoxyglucose positron emission tomography scans classically show frontal and/or anterior temporal hypometabolism, which helps differentiate the disease from Alzheimer's disease, as the PET scan in Alzheimer's disease classically shows biparietal hypometabolism.
Meta-analyses based on imaging methods have shown that frontotemporal dementia mainly affects a frontomedial network discussed in the context of social cognition or "theory of mind".[45] This is entirely in keeping with the notion that on the basis of cognitive neuropsychological evidence, the ventromedial prefrontal cortex is a major locus of dysfunction early on in the course of the behavioural variant of frontotemporal degeneration.[46] The language subtypes of FTLD (semantic dementia and progressive nonfluent aphasia) can be regionally dissociated by imaging approaches in vivo.[47]
The confusion between Alzheimer's and FTD is justifiable due to the similarities between their initial symptoms. Patients do not have difficulty with movement and other motor tasks.[48] As FTD symptoms appear, it is difficult to differentiate between a diagnosis of Alzheimer's disease and FTD. There are distinct differences in the behavioral and emotional symptoms of the two dementias, notably, the blunting of affect seen in FTD patients.[13] In the early stages of FTD, anxiety and depression are common, which may result in an ambiguous diagnosis. However, over time, these ambiguities fade away as this dementia progresses and defining symptoms of apathy, unique to FTD, start to appear.[citation needed]
Recent studies over several years have developed new criteria for the diagnosis of behavioral variant frontotemporal dementia (bvFTD). The confirmatory diagnosis is made by brain biopsy, but other tests can be used to help, such as MRI, EEG, CT, and physical examination and history.[49] As of 2011[update], six distinct clinical features have been identified as symptoms of bvFTD.[50]
- Disinhibition
- Apathy / Inertia
- Loss of Sympathy / Empathy
- Perseverative / Compulsive behaviors
- Hyperorality
- Dysexecutive neuropsychological profile
Of the six features, three must be present in a patient to diagnose one with possible bvFTD. Similar to standard FTD, the primary diagnosis stems from clinical trials that identify the associated symptoms, instead of imaging studies.[50] The above criteria are used to distinguish bvFTD from disorders such as Alzheimer's and other causes of dementia. In addition, the criteria allow for a diagnostic hierarchy distinguished possible, probable, and definite bvFTD based on the number of symptoms present.[50]
A 2021 study, determined that using cerebrospinal fluid (CSF) biomarkers of pathologic amyloid plaques, tangles, and neurodegeneration, collectively called ATN, can be useful in diagnosing FTD.[51]
Neuropsychological tests[edit]
The progression of the degeneration caused by bvFTD may follow a predictable course. The degeneration begins in the orbitofrontal cortex and medial aspects such as ventromedial prefrontal cortex. In later stages, it gradually expands its area to the dorsolateral prefrontal cortex and the temporal lobe.[52] Thus, the detection of dysfunction of the orbitofrontal cortex and ventromedial cortex is important in the detection of early stage bvFTD. As stated above, a behavioural change may occur before the appearance of any atrophy in the brain in the course of the disease. Because of that, image scanning such as MRI can be insensitive to the early degeneration and it is difficult to detect early-stage bvFTD.[citation needed]
In neuropsychology, there is an increasing interest in using neuropsychological tests such as the Iowa gambling task or Faux Pas Recognition test as an alternative to imaging for the diagnosis of bvFTD.[53] Both the Iowa gambling task and the Faux Pas test are known to be sensitive to dysfunction of the orbitofrontal cortex.[citation needed]
The Faux Pas Recognition test is intended to measure one's ability to detect faux pas types of social blunders (accidentally making a statement or an action that offends others). It is suggested that people with orbitofrontal cortex dysfunction show a tendency to make social blunders due to a deficit in self-monitoring.[54] Self-monitoring is the ability of individuals to evaluate their own behavior to make sure that their behavior is appropriate in particular situations. The impairment in self-monitoring leads to a lack of social emotion signals. The social emotions such as embarrassment are important in the way that they alert the individual to adapt social behavior in an appropriate manner to maintain relationships with others. Though patients with damage to the OFC retain intact knowledge of social norms, they fail to apply it to actual behavior, because they fail to generate social emotions that promote adaptive social behavior.[54]
The other test, the Iowa gambling task, is a psychological test intended to simulate real-life decision making. The underlying concept of this test is the somatic marker hypothesis. This hypothesis argues that when people have to make complex uncertain decisions, they employ both cognitive and emotional processes to assess the values of the choices available to them. Each time a person makes a decision, both physiological signals and evoked emotion (somatic markers) are associated with their outcomes, and this accumulates as experience. People tend to choose the choice which might produce the outcome reinforced with positive stimuli; thus it biases decision-making towards certain behaviors while avoiding others.[55] It is thought that somatic markers are processed in the orbitofrontal cortex.[citation needed]
The symptoms observed in bvFTD are caused by dysfunction of the orbitofrontal cortex; thus these two neuropsychological tests might be useful in detecting early-stage bvFTD. However, as self-monitoring and somatic marker processes are so complex, it likely involves other brain regions. Therefore, neuropsychological tests are sensitive to the dysfunction of orbitofrontal cortex, yet are not specific to it. The weakness of these tests is that they do not necessarily show dysfunction of the orbitofrontal cortex.[citation needed]
In order to solve this problem, some researchers have combined neuropsychological tests which detect the dysfunction of orbitofrontal cortex into one grouping, so that it increases its specificity to the degeneration of the frontal lobe, in order to detect early-stage bvFTD. They invented the Executive and Social Cognition Battery which comprises five neuropsychological tests:[53]
- Faux Pas test
- Hotel task
- Iowa gambling task
- Mind in the Eyes
- Multiple Errands task
The result has shown that this combined test is more sensitive in detecting the deficits in early bvFTD.[53]
Management[edit]
Currently, there is no cure for FTD. Treatments are available to manage the behavioral symptoms. Disinhibition and compulsive behaviors can be controlled by selective serotonin reuptake inhibitors (SSRIs).[56][57] Agitation can be controlled with small doses of atypical antipsychotics.[58] Although Alzheimer's and FTD share certain symptoms, they cannot be treated with the same pharmacological agents because the cholinergic systems are not affected in FTD.[13]
Because FTD often occurs in relatively younger adults (i.e. in their 40s or 50s), it can severely affect families. Patients often still have children living in the home.[citation needed]
Prognosis[edit]
Symptoms of frontotemporal dementia progress at a rapid, steady rate. Patients with the disease can survive for 2–20 years. Eventually patients will need 24-hour care for daily function.[59]
Cerebrospinal fluid leaks are a known cause of reversible frontotemporal dementia.[60]
History[edit]
Features of FTD were first described by the Czech psychiatrist Arnold Pick between 1892 and 1906.[9][61] The name Pick's disease was coined in 1922.[10] This term is now reserved only for behavioral variant FTD which shows the presence of the characteristic Pick bodies and Pick cells,[11][12] which were first described by Alois Alzheimer in 1911.[10]
In 1989, Snowden suggested the term semantic dementia to describe the patient with predominant left temporal atrophy and aphasia that Pick described. The first research criteria for FTD, "Clinical and neuropathological criteria for frontotemporal dementia. The Lund and Manchester Groups", was developed in 1994. The clinical diagnostic criteria were revised in the late 1990s, when the FTD spectrum was divided into a behavioral variant, a nonfluent aphasia variant, and a semantic dementia variant.[20] The most recent revision of the clinical research criteria was by the International Behavioural Variant FTD Criteria Consortium in 2011.[50]
Notable cases[edit]
People who have been diagnosed as having FTD (often referred to as Pick's disease in cases of the behavioral variant) include:
- John Berry (1963–2016), American hardcore punk musician and founding member of the Beastie Boys[62]
- Clancy Blair (born 1960), American developmental psychologist and professor[citation needed]
- Don Cardwell (1935–2008), Major League Baseball pitcher[63]
- Charmian Carr (1942–2016), who played Liesl, from the Sound of Music, born Charmian Anne Farnon
- Jerry Corbetta (1947–2016), frontman, organist and keyboardist of American psychedelic rock band Sugarloaf[64]
- Ted Darling (1935–1996), Buffalo Sabres television announcer
- Robert W. Floyd (1936–2001), computer scientist[65]
- Lee Holloway (born 1982), computer scientist, co-founder of Cloudflare[66]
- Colleen Howe (1933–2009), sports agent and ice hockey team manager, known as "Mrs. Hockey"[67]
- Kazi Nazrul Islam (1899–1976) national poet of Bangladesh[68]
- Terry Jones (1942–2020), Welsh comedian (Monty Python) and director
- Ralph Klein (1942–2013), former premier of Alberta, Canada
- Kevin Moore (1958–2013), English footballer[69]
- Ernie Moss (1949–2021), English footballer[70]
- Nic Potter (1951–2013), British bassist for Van der Graaf Generator[71]
- Christina Ramberg (1946–1995), American painter associated with the Chicago Imagists[72]
- David Rumelhart (1942–2011), American cognitive psychologist[citation needed]
- Sir Nicholas Wall (1945–2017), English judge[73]
- Wendy Williams (born 1964), American broadcaster[74]
- Bruce Willis (born 1955), American actor [75]
- Mark Wirtz (1943–2020), pop musician, composer and producer[76]
See also[edit]
References[edit]
- ^ a b c d Olney NT, Spina S, Miller BL (May 2017). "Frontotemporal dementia". Neurologic Clinics. 35 (2): 339–374. doi:10.1016/j.ncl.2017.01.008. PMC 5472209. PMID 28410663.
- ^ Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Arlington, VA: American Psychiatric Association. 2013. pp. 614–618. ISBN 978-0-89042-554-1.
- ^ "ICD-11 – Mortality and Morbidity Statistics". icd.who.int.
- ^ a b Sivasathiaseelan H, Marshall CR, Agustus JL, Benhamou E, Bond RL, van Leeuwen JE, et al. (April 2019). "Frontotemporal Dementia: A Clinical Review". Seminars in Neurology. 39 (2): 251–263. doi:10.1055/s-0039-1683379. PMID 30925617. S2CID 88481297.
- ^ "Frontotemporal dementia". Johns Hopkins Medicine. 2024. Retrieved 23 March 2024.
- ^ a b Borroni B, Graff C, Hardiman O, Ludolph AC, Moreno F, Otto M, et al. (March 2022). "FRONTotemporal dementia Incidence European Research Study-FRONTIERS: Rationale and design". Alzheimer's & Dementia. 18 (3): 498–506. doi:10.1002/alz.12414. PMC 9291221. PMID 34338439.
- ^ a b Hofmann JW, Seeley WW, Huang EJ (January 2019). "RNA Binding Proteins and the Pathogenesis of Frontotemporal Lobar Degeneration". Annual Review of Pathology. 14: 469–495. doi:10.1146/annurev-pathmechdis-012418-012955. PMC 6731550. PMID 30355151.
- ^ Chen I (June 2009). "Brain Cells for Socializing". Smithsonian. Retrieved 30 October 2015.
- ^ a b Mikol J (2018). "History of Pick's disease". Revue Neurologique. 174 (10): 740–741. doi:10.1016/j.neurol.2018.09.009. ISSN 0035-3787. S2CID 81923630.
- ^ a b c d Pearce JM (February 2003). "Pick's disease". Journal of Neurology, Neurosurgery, and Psychiatry. 74 (2): 169. doi:10.1136/jnnp.74.2.169. PMC 1738259. PMID 12531941.
- ^ a b Ropper AH, Samuels MA, Klein JP (2019). Adams and Victor's Principles of Neurology (eleventh ed.). McGraw Hill. p. 1096. ISBN 978-0-07-184262-4.
- ^ a b c Cardarelli R, Kertesz A, Knebl JA (December 2010). "Frontotemporal dementia: a review for primary care physicians". American Family Physician. 82 (11): 1372–1377. PMID 21121521.
- ^ a b c d Snowden JS, Neary D, Mann DM (February 2002). "Frontotemporal dementia". The British Journal of Psychiatry. 180 (2): 140–143. doi:10.1192/bjp.180.2.140. PMID 11823324.
- ^ a b Rabinovici GD, Miller BL (May 2010). "Frontotemporal lobar degeneration: epidemiology, pathophysiology, diagnosis and management". CNS Drugs. 24 (5): 375–398. doi:10.2165/11533100-000000000-00000. PMC 2916644. PMID 20369906.
- ^ a b c d e Finger EC (April 2016). "Frontotemporal Dementias". Continuum. 22 (2 Dementia): 464–489. doi:10.1212/CON.0000000000000300. PMC 5390934. PMID 27042904.
- ^ "Focus on Frontotemporal Dementia (FTD)". National Institute of Neurological Disorders and Stroke. U.S. National Institutes of Health. Archived from the original on 16 October 2021. Retrieved 3 March 2021.
- ^ Sartorius N, Henderson A, Strotzka H, Lipowski Z, Yu-cun S, You-xin X, et al. (1992). The ICD-10 Classification of Mental and Behavioural Disorders Clinical descriptions and diagnostic guidelines (PDF). World Health Organization. p. 51. ISBN 92-4-154422-8.
- ^ a b "What is frontotemporal dementia". Dementia UK. Retrieved 2020-10-19.
- ^ "What are the Different Types of Frontotemporal Disorders?". National Institute on Aging. Retrieved 1 November 2020.
- ^ a b Olney NT, Spina S, Miller BL (May 2017). "Frontotemporal Dementia". Neurologic Clinics. 35 (2): 339–374. doi:10.1016/j.ncl.2017.01.008. PMC 5472209. PMID 28410663.
- ^ "Frontotemporal Dementia Information Page". National Institute of Neurological Disorders and Stroke. U.S. National Institutes of Health.
- ^ a b c d Sleegers K, Cruts M, Van Broeckhoven C (2010). "Molecular pathways of frontotemporal lobar degeneration". Annual Review of Neuroscience. 33 (1): 71–88. doi:10.1146/annurev-neuro-060909-153144. PMID 20415586.
- ^ Bang J, Spina S, Miller BL (October 2015). "Frontotemporal dementia". Lancet. 386 (10004): 1672–1682. doi:10.1016/S0140-6736(15)00461-4. PMC 5970949. PMID 26595641.
- ^ Gaillard F. "Pick bodies". Radiology Reference Article. Radiopaedia. Retrieved 12 March 2021.
- ^ a b c Cairns NJ, Grossman M, Arnold SE, Burn DJ, Jaros E, Perry RH, et al. (October 2004). "Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease". Neurology. 63 (8): 1376–1384. doi:10.1212/01.wnl.0000139809.16817.dd. PMC 3516854. PMID 15505152.
- ^ a b Armstrong RA, Gearing M, Bigio EH, Cruz-Sanchez FF, Duyckaerts C, Mackenzie IR, et al. (November 2011). "Spatial patterns of FUS-immunoreactive neuronal cytoplasmic inclusions (NCI) in neuronal intermediate filament inclusion disease (NIFID)". Journal of Neural Transmission. 118 (11): 1651–1657. doi:10.1007/s00702-011-0690-x. PMC 3199334. PMID 21792670.
- ^ Ito H (December 2014). "Basophilic inclusions and neuronal intermediate filament inclusions in amyotrophic lateral sclerosis and frontotemporal lobar degeneration". Neuropathology. 34 (6): 589–595. doi:10.1111/neup.12119. PMID 24673472. S2CID 19275295.
- ^ Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, et al. (November 2009). "FUS pathology in basophilic inclusion body disease". Acta Neuropathologica. 118 (5): 617–627. doi:10.1007/s00401-009-0598-9. hdl:2429/54671. PMID 19830439. S2CID 22541167.
- ^ a b Piguet O (November 2011). "Eating disturbance in behavioural-variant frontotemporal dementia". Journal of Molecular Neuroscience. 45 (3): 589–593. doi:10.1007/s12031-011-9547-x. PMID 21584651. S2CID 24125998.
- ^ Neary D, Snowden J, Mann D (November 2005). "Frontotemporal dementia". The Lancet. Neurology. 4 (11): 771–780. doi:10.1016/S1474-4422(05)70223-4. PMID 16239184. S2CID 17310802.
- ^ Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, et al. (December 2003). "Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease". Cognitive and Behavioral Neurology. 16 (4): 211–218. doi:10.1097/00146965-200312000-00002. PMID 14665820. S2CID 46800951.
- ^ Olney RK, Murphy J, Forshew D, Garwood E, Miller BL, Langmore S, et al. (December 2005). "The effects of executive and behavioral dysfunction on the course of ALS". Neurology. 65 (11): 1774–1777. doi:10.1212/01.wnl.0000188759.87240.8b. PMID 16344521. S2CID 11672234.
- ^ Buée L, Delacourte A (October 1999). "Comparative biochemistry of tau in progressive supranuclear palsy, corticobasal degeneration, FTDP-17 and Pick's disease". Brain Pathology. 9 (4): 681–693. doi:10.1111/j.1750-3639.1999.tb00550.x. PMC 8098140. PMID 10517507. S2CID 10711305.
- ^ Hardy J, Momeni P, Traynor BJ (April 2006). "Frontal temporal dementia: dissecting the aetiology and pathogenesis". Brain. 129 (Pt 4): 830–831. doi:10.1093/brain/awl035. PMID 16543401.
- ^ Darwich NF, Phan JM, Kim B, Suh E, Papatriantafyllou JD, Changolkar L, et al. (November 2020). "Autosomal dominant VCP hypomorph mutation impairs disaggregation of PHF-tau". Science. 370 (6519): eaay8826. doi:10.1126/science.aay8826. PMC 7818661. PMID 33004675.
- ^ Convery R, Mead S, Rohrer JD (February 2019). "Review: Clinical, genetic and neuroimaging features of frontotemporal dementia". Neuropathology and Applied Neurobiology. 45 (1): 6–18. doi:10.1111/nan.12535. PMID 30582889. S2CID 58636022.
- ^ van Blitterswijk M, DeJesus-Hernandez M, Rademakers R (December 2012). "How do C9ORF72 repeat expansions cause amyotrophic lateral sclerosis and frontotemporal dementia: can we learn from other noncoding repeat expansion disorders?". Current Opinion in Neurology. 25 (6): 689–700. doi:10.1097/WCO.0b013e32835a3efb. PMC 3923493. PMID 23160421.
- ^ Abugable AA, Morris JL, Palminha NM, Zaksauskaite R, Ray S, El-Khamisy SF (September 2019). "DNA repair and neurological disease: From molecular understanding to the development of diagnostics and model organisms". DNA Repair. 81: 102669. doi:10.1016/j.dnarep.2019.102669. PMID 31331820.
- ^ Dobson-Stone C, Hallupp M, Shahheydari H, Ragagnin AM, Chatterton Z, Carew-Jones F, et al. (March 2020). "CYLD is a causative gene for frontotemporal dementia - amyotrophic lateral sclerosis". Brain. 143 (3): 783–799. doi:10.1093/brain/awaa039. PMC 7089666. PMID 32185393.
- ^ Feng T, Lacrampe A, Hu F (March 2021). "Physiological and pathological functions of TMEM106B: a gene associated with brain aging and multiple brain disorders". Acta Neuropathologica. 141 (3): 327–339. doi:10.1007/s00401-020-02246-3. PMC 8049516. PMID 33386471.
- ^ Liscic RM, Storandt M, Cairns NJ, Morris JC (April 2007). "Clinical and psychometric distinction of frontotemporal and Alzheimer dementias". Archives of Neurology. 64 (4): 535–540. doi:10.1001/archneur.64.4.535. PMID 17420315.
- ^ Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. (September 2011). "Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration". Brain. 134 (Pt 9): 2565–2581. doi:10.1093/brain/awr198. PMC 3170537. PMID 21908872.
- ^ van der Zee J, Van Broeckhoven C (February 2014). "Dementia in 2013: frontotemporal lobar degeneration-building on breakthroughs". Nature Reviews. Neurology. 10 (2): 70–72. doi:10.1038/nrneurol.2013.270. PMID 24394289.
- ^ Wang H, Kodavati M, Britz GW, Hegde ML (2021). "DNA Damage and Repair Deficiency in ALS/FTD-Associated Neurodegeneration: From Molecular Mechanisms to Therapeutic Implication". Frontiers in Molecular Neuroscience. 14: 784361. doi:10.3389/fnmol.2021.784361. PMC 8716463. PMID 34975400.
- ^ Schroeter ML, Raczka K, Neumann J, von Cramon DY (March 2008). "Neural networks in frontotemporal dementia--a meta-analysis". Neurobiology of Aging. 29 (3): 418–426. doi:10.1016/j.neurobiolaging.2006.10.023. PMID 17140704. S2CID 9039002.
- ^ Rahman S, Sahakian BJ, Hodges JR, Rogers RD, Robbins TW (August 1999). "Specific cognitive deficits in mild frontal variant frontotemporal dementia". Brain. 122 (8): 1469–1493. doi:10.1093/brain/122.8.1469. PMID 10430832.
- ^ Schroeter ML, Raczka K, Neumann J, Yves von Cramon D (July 2007). "Towards a nosology for frontotemporal lobar degenerations-a meta-analysis involving 267 subjects". NeuroImage. 36 (3): 497–510. doi:10.1016/j.neuroimage.2007.03.024. PMID 17478101. S2CID 130161.
- ^ Steinbart EJ, Smith CO, Poorkaj P, Bird TD (November 2001). "Impact of DNA testing for early-onset familial Alzheimer disease and frontotemporal dementia". Archives of Neurology. 58 (11): 1828–1831. doi:10.1001/archneur.58.11.1828. PMID 11708991.
- ^ MedlinePlus Encyclopedia: Frontotemporal dementia
- ^ a b c d Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. (September 2011). "Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia". Brain. 134 (Pt 9): 2456–2477. doi:10.1093/brain/awr179. PMC 3170532. PMID 21810890.
- ^ Cousins KA, Phillips JS, Irwin DJ, Lee EB, Wolk DA, Shaw LM, et al. (May 2021). "ATN incorporating cerebrospinal fluid neurofilament light chain detects frontotemporal lobar degeneration". Alzheimer's & Dementia. 17 (5): 822–830. doi:10.1002/alz.12233. PMC 8119305. PMID 33226735.
- ^ Krueger CE, Bird AC, Growdon ME, Jang JY, Miller BL, Kramer JH (August 2009). "Conflict monitoring in early frontotemporal dementia". Neurology. 73 (5): 349–355. doi:10.1212/wnl.0b013e3181b04b24. PMC 2725928. PMID 19652138.
- ^ a b c Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F (May 2009). "A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia". Brain. 132 (Pt 5): 1299–1309. doi:10.1093/brain/awp041. PMID 19336463.
- ^ a b Beer JS, John OP, Scabini D, Knight RT (June 2006). "Orbitofrontal cortex and social behavior: integrating self-monitoring and emotion-cognition interactions". Journal of Cognitive Neuroscience. 18 (6): 871–879. CiteSeerX 10.1.1.527.3607. doi:10.1162/jocn.2006.18.6.871. PMID 16839295. S2CID 13590871.
- ^ Damasio AR (October 1996). "The somatic marker hypothesis and the possible functions of the prefrontal cortex". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 351 (1346): 1413–1420. doi:10.1098/rstb.1996.0125. PMID 8941953. S2CID 1841280.
- ^ Swartz JR, Miller BL, Lesser IM, Darby AL (May 1997). "Frontotemporal dementia: treatment response to serotonin selective reuptake inhibitors". The Journal of Clinical Psychiatry. 58 (5): 212–216. doi:10.4088/jcp.v58n0506. PMID 9184615.
- ^ "Medications for behavioral symptoms". Weill Institute for Neurosciences. University of California San Francisco. Retrieved 30 October 2015.
- ^ Manoochehri M, Huey ED (October 2012). "Diagnosis and management of behavioral issues in frontotemporal dementia". Current Neurology and Neuroscience Reports. 12 (5): 528–536. doi:10.1007/s11910-012-0302-7. PMC 3443960. PMID 22847063.
- ^ Kertesz A (June 2004). "Frontotemporal dementia/Pick's disease". Archives of Neurology. 61 (6): 969–971. doi:10.1001/archneur.61.6.969. PMID 15210543.
- ^ Samson K (2002). "Hypotension May Cause Frontotemporal Dementia". Neurology Today. 2 (9): 35–36. doi:10.1097/00132985-200209000-00013.
- ^ Pick A (1892). "Uber die Beziehungen der senilen Hirnatrophie zur Aphasie". Prag Med Wochenschr. 17: 165–167.
- ^ Oyebode J (31 May 2016). "Beastie Boy John Berry Died of Frontal Lobe Dementia – But What Is It?". Elsevier. Retrieved 10 May 2022.
- ^ Goldstein R (16 January 2008). "Don Cardwell, 72, Pitcher for 1969 Mets, Is Dead". The New York Times.
- ^ "Sugarloaf frontman Jerry Corbetta dead at 68". The Denver Post. 2016-09-20. Retrieved 2016-10-29.
- ^ Knuth DE (December 2003). "Robert W Floyd, In Memoriam". ACM SIGACT News. 34 (4): 3–13. doi:10.1145/954092.954488. S2CID 35605565.
- ^ Upson S (15 April 2020). "The Devastating Decline of a Brilliant Young Coder". Wired.
- ^ Sipple G (March 6, 2009). "Fans mourn loss of "Mrs. Hockey," Colleen Howe". The Detroit Free Press. Archived from the original on October 4, 2015. Retrieved October 3, 2015.
- ^ Farooq MO. "Kazi Nazrul Islam: Illness and Treatment". nazrul.org. Archived from the original on 29 May 2015. Retrieved 26 March 2016.
- ^ Sportsteam. "Grimsby Town legend Kevin Moore passes away". Archived from the original on 2013-05-03. Retrieved 2013-04-29.
- ^ "Support for Valiants hero Ernie Moss after he is diagnosed with Pick's Disease". The Sentinel. 11 October 2014. Archived from the original on 2 November 2014. Retrieved 11 October 2014.
- ^ Potter S (22 January 2013). "Nic Potter obituary | Music | guardian.co.uk". London: Guardian. Retrieved 22 January 2013.
- ^ Cook G (27 February 2014). "The Sexy, Proto-Feminist Art Of Christina Ramberg's Tragically Short Life". www.wbur.org.
- ^ Rayner G (23 February 2017). "Sir Nicholas Wall, once Britain's top family law judge, commits suicide after dementia diagnosis". The Daily Telegraph.
- ^ Shah S (22 February 2024). "Wendy Williams Diagnosed With Frontotemporal Dementia and Apahasia". TIME. Retrieved 22 February 2024.
- ^ Moreau J (2023-02-16). "Bruce Willis Diagnosed With Dementia After Retiring Due to Aphasia". Variety. Retrieved 2023-02-16.
- ^ "Mark Wirtz, musician and producer who had a global hit with Excerpt from a Teenage Opera – obituary". The Daily Telegraph. 13 August 2020. Archived from the original on 27 August 2020.
Further reading[edit]
- Liu W, Miller BL, Kramer JH, Rankin K, Wyss-Coray C, Gearhart R, et al. (March 2004). "Behavioral disorders in the frontal and temporal variants of frontotemporal dementia". Neurology. 5. 62 (5): 742–748. doi:10.1212/01.WNL.0000113729.77161.C9. PMC 2367136. PMID 15007124.
- Nyatsanza S, Shetty T, Gregory C, Lough S, Dawson K, Hodges JR (October 2003). "A study of stereotypic behaviours in Alzheimer's disease and frontal and temporal variant frontotemporal dementia". Journal of Neurology, Neurosurgery, and Psychiatry. 74 (10): 1398–1402. doi:10.1136/jnnp.74.10.1398. PMC 1757381. PMID 14570833.
- Hsiung GY, Feldman HH (1993). "GRN Frontotemporal Dementia". In Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJ, Gripp KW, Amemiya A (eds.). GeneReviews. Seattle: University of Washington. PMID 20301545.
- Rohrer J, Ryan B, Ahmed R (1993). "MAPT-Related Frontotemporal Dementia". In Adam MP, Mirzaa GM, Pagon RA, et al. (eds.). GeneReviews. Seattle: University of Washington, Seattle. PMID 20301678.
- Reynolds M. "'Til Death or Dementia Do us Part, a memoir". River Rock Books. Archived from the original on 2018-02-20. Retrieved 2018-02-19.
- Upson S (15 April 2020). "The Devastating Decline of a Brilliant Young Coder". Wired. Retrieved 17 September 2020.
