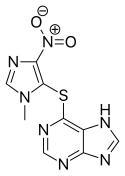
Azathioprine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌæzəˈθaɪəˌpriːn/[1] |
| Trade names | Azasan, Imuran, Jayempi, others |
| Other names | AZA |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682167 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 60±31% |
| Protein binding | 20–30% |
| Metabolism | Activated non-enzymatically, deactivated mainly by xanthine oxidase |
| Elimination half-life | 26–80 minutes (azathioprine) 3–5 hours (drug plus metabolites) |
| Excretion | Kidney, 98% as metabolites |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.525 |
| Chemical and physical data | |
| Formula | C9H7N7O2S |
| Molar mass | 277.26 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 238 to 245 °C (460 to 473 °F) |
| |
| |
| (verify) | |
Azathioprine, sold under the brand name Imuran, among others, is an immunosuppressive medication.[5] It is used for the treatment of rheumatoid arthritis, granulomatosis with polyangiitis, Crohn's disease, ulcerative colitis, and systemic lupus erythematosus; and in kidney transplants to prevent rejection. It is listed by the International Agency for Research on Cancer as a group 1 human carcinogen.[5][6][7][8] It is taken by mouth or injected into a vein.[5]
Common side effects include bone-marrow suppression and vomiting.[5] Bone-marrow suppression is especially common in people with a genetic deficiency of the enzyme thiopurine S-methyltransferase.[5] Other serious risk factors include an increased risk of certain cancers.[5] Use during pregnancy may result in harm to the baby.[5] Azathioprine belongs to the purine analogues subclass of antimetabolites family of medications.[5][9] It works via 6-thioguanine to disrupt the making of RNA and DNA by cells.[5][9]
Azathioprine was first made in 1957.[9] It is on the World Health Organization's List of Essential Medicines.[10] In 2018, it was the 358th most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[11]
Medical uses
[edit]Azathioprine is used alone or in combination with other immunosuppressive therapy to prevent rejection following organ transplantation, and to treat an array of autoimmune diseases, including rheumatoid arthritis, pemphigus, systemic lupus erythematosus, Behçet's disease, and other forms of vasculitis, autoimmune hepatitis, atopic dermatitis, myasthenia gravis, neuromyelitis optica (Devic's disease), restrictive lung disease, and others.[12] It is also an important therapy and steroid-sparing agent for inflammatory bowel disease (such as Crohn's disease and ulcerative colitis) and for multiple sclerosis.[13]
In the United States, it is approved by the Food and Drug Administration for use in kidney transplantation from human donors, and for rheumatoid arthritis.[14]
Transplantation
[edit]Azathioprine is used to prevent rejections of kidney or liver allografts, usually in conjunction with other therapies including corticosteroids, other immunosuppressants, and local radiation therapy.[15][16] The administration protocol starts either at the time of transplantation or within the following two days.[14]
Rheumatoid arthritis
[edit]Being a disease-modifying antirheumatic drug (DMARD), azathioprine has been used for the management of the signs and symptoms of adult rheumatoid arthritis.[17] Nonsteroidal anti-inflammatory drugs and corticosteroids may be combined or continued (if they were already in use) with azathioprine, but the combination with other DMARDs is not recommended.[14]
Inflammatory bowel disease
[edit]Azathioprine has been used in the management of moderate to severe chronically active Crohn's disease,[18] to maintain clinical remission (absence of disease activity) in corticosteroid-dependent patients,[19] and to provide benefit in people with fistulizing Crohn's disease.[20] The onset of action is slow, and it may require several months to achieve clinical response.[18]
Azathioprine treatment is associated with an increased risk of lymphoma, but if this is due to the drug or a predisposition related to Crohn's disease is unclear.[21] Lower doses of azathioprine are used as a therapy in children with refractory or corticosteroid-dependent Crohn's disease, without causing many side effects.[22] It may also be used to prevent flares in those with ulcerative colitis.[23]
Others
[edit]Azathioprine is sometimes used in systemic lupus erythematosus, requiring a maintenance dose of 15 mg or higher of prednisone in those who experience recurrent flares.[24]
It is used as an add-on therapy when steroid therapy is given by mouth for pemphigus and myasthenia gravis, as a "steroid-sparing" agent.[12][25][26] Azathioprine is also used to maintain remission in people who have granulomatosis with polyangiitis.[7]
It can be very effective in eczema and atopic dermatitis, though it is not commonly used.[12] The British National Eczema Society lists it as a third-line treatment for severe to moderate cases of these skin diseases.[27]
It was widely used for the treatment of multiple sclerosis until the first half of the 1990s. Concerns about increased risk of malignancy has led to a decreased use, yet it is still used in maintenance treatment for people who frequently relapse.[28] A 2007 Cochrane review found that azathioprine reduced the number of relapses in the first year of treatment and disease progression in the first two to three years and did not find an increase in cancer, and noted the need for direct comparison of azathioprine and interferon beta, conflicting conclusions regarding cancer, and the potential for long-term risks.[29]
A widely used therapy for idiopathic pulmonary fibrosis was azathioprine in combination with prednisone and N-acetylcysteine. A 2012 study showed that outcomes were worse with this combination than with placebo.[30]
Adverse effects
[edit]
Nausea and vomiting are common adverse effects, especially at the beginning of a treatment. Such cases are met with taking azathioprine after meals or transient intravenous administration. Side effects that are probably hypersensitivity reactions include dizziness, diarrhea, fatigue, and rashes. Hair loss is often seen in transplant patients receiving the drug, but rarely occurs under other indications. Because azathioprine suppresses the bone marrow, patients can develop anaemia and be more susceptible to infection; regular monitoring of the blood count is recommended during treatment.[14][31] Acute pancreatitis can also occur, especially in patients with Crohn's disease.[32] Treatment is discontinued in up to 30% of patients due these effects but therapeutic drug monitoring of the biologically active metabolites, i.e. thiopurine nucleotides can help to optimize the efficacy and safety. Clinically, most hospitals resort to ion-exchange LC-MS (liquid chromotography – mass spectrometry) but the newly developed approach of porous graphitic carbon based chromatography hyphenated with mass spectrometry appears superior with respect to patient care in this respect.[33]
It is listed by the International Agency for Research on Cancer as a group 1 carcinogen (carcinogenic to humans).[34]
Pharmacogenetics
[edit]The enzyme thiopurine S-methyltransferase (TPMT) is responsible for various activation and deactivation steps in azathioprine's mechanism of action.[35] The first metabolic step that azathioprine undergoes in the body is the conversion to 6-mercaptopurine (6-MP; see Pharmacokinetics), which is itself an immunosuppressant prodrug.[36][37] The TPMT enzyme is responsible, in part, for the methylation of 6-MP into the inactive metabolite 6-methylmercaptopurine – this methylation prevents 6-MP from further conversion into active, cytotoxic thioguanine nucleotide (TGN) metabolites.[36][38] Certain genetic variations within the TPMT gene can lead to decreased or absent TPMT enzyme activity, and individuals who are homozygous or heterozygous for these types of genetic variations may have increased levels of TGN metabolites and an increased risk of severe bone marrow suppression (myelosuppression) when receiving azathioprine.[39] In many ethnicities, TPMT polymorphisms that result in decreased or absent TPMT activity occur with a frequency of approximately 5%, meaning that about 0.25% of patients are homozygous for these variants.[39][40] However, an assay of TPMT activity in red blood cells or a TPMT genetic test can identify patients with reduced TPMT activity, allowing for the adjustment of azathioprine dose or avoidance of the drug entirely.[39][41] The FDA-approved drug label for azathioprine recommends testing for TPMT activity to identify patients at risk for myelotoxicity.[42] Indeed, testing for TPMT activity is one of the few examples of pharmacogenetics being translated into routine clinical care.[43] Missense SNP in NUDT15 (e.g., rs116855232, inducing R139C)) has been identified to be a causal factor for AZA-induced leukopenia through a genome wide association study (GWAS) in East Asians.[44]
Cancers
[edit]Azathioprine is listed as a human carcinogen in the 12th Report on Carcinogens by the National Toxicology Program of U.S. Department of Health and Human Services, asserting that it is "known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans."[45] Since August 2009, the U.S. FDA has required warnings to be placed on packaging with respect to increased risks of certain cancers.[46]
The risks involved seem to be related both to the duration and the dosage used. People who have previously been treated with an alkylating agent may have an excessive risk of cancers if treated with azathioprine. Epidemiological studies by International Agency for Research on Cancer have provided "sufficient" evidence of azathioprine carcinogenicity in humans (group 1),[47] although the methodology of past studies and the possible underlying mechanisms are questioned.[48]
The various diseases requiring transplantation may in themselves increase the risks of non-Hodgkin lymphoma, squamous cell carcinomas of the skin, hepatobiliary carcinomas, and mesenchymal tumours to which azathioprine may add additional risks. Those receiving azathioprine for rheumatoid arthritis may have a lower risk than those undergoing transplantation.[34]
Cases of hepatosplenic T-cell lymphoma – a rare type of lymphoma – have been reported in patients treated with azathioprine. The majority occurred in patients with inflammatory bowel disease. Adolescents and young adult males were the majority of cases.[49] They presented with a very aggressive disease course, and with one exception, died of the lymphoma. The FDA has required changes to the labeling to inform users and clinicians of the issue.[50]
Skin cancers
[edit]In transplant patients, skin cancer is 50 to 250 times more common than in the general population, and between 60 and 90% of patients are affected 20 years after transplantation. The use of immunosuppressive medication including azathioprine in organ transplantation has been linked to increased rates of developing skin cancer.[51] Azathioprine causes the accumulation of 6-thioguanine (6-TG) in patients' DNA, which might trigger cancer when the patient is later exposed to ultraviolet light. Patients taking azathioprine were found to be abnormally sensitive to UVA light.[52]
Overdose
[edit]Large single doses are generally well tolerated; a patient who took 7.5 g azathioprine (150 tablets) at once showed no relevant symptoms apart from vomiting, slightly decreased white blood cell count, and marginal changes in liver function parameters. Main symptoms of long-term overdosing are infections of unclear origin, mouth ulcers, and spontaneous bleeding, all of which are consequences of its bone-marrow suppression.[31]
Interactions
[edit]Other purine analogues, such as allopurinol, inhibit xanthine oxidase, the enzyme that breaks down azathioprine, thus increasing the toxicity of azathioprine.[53] Low doses of allopurinol, though, have been shown to safely enhance the efficacy of azathioprine, especially in inflammatory bowel disease nonresponders.[54][55][56] This may still lead to lower lymphocyte counts and higher rates of infection, therefore the combination requires careful monitoring.[57][58]
Azathioprine decreases the effects of the anticoagulant warfarin and of nondepolarizing muscle relaxants, but increases the effect of depolarizing muscle relaxants.[31] It can also interfere with niacin (vitamin B3), resulting in at least one case to pellagra and fatal medullary aplasia.[59]
Pregnancy and breastfeeding
[edit]Azathioprine can cause birth defects.[60][61][62] A 2003 population-based study in Denmark showed that the use of azathioprine and related mercaptopurine resulted in a seven-fold incidence of fetal abnormalities, as well as a 20-fold increase in miscarriage.[63] Birth defects in a child whose father was taking azathioprine have also been reported.[64] Although no adequate and well-controlled studies have taken place in humans, when given to animals in doses equivalent to human dosages, teratogenesis was observed.[65] Transplant patients already on this drug should not discontinue on becoming pregnant. This contrasts with the later-developed drugs tacrolimus and mycophenolate, which are contraindicated during pregnancy.[60]
Traditionally, as for all cytotoxic drugs, the manufacturer advises not to breastfeed whilst taking azathioprine, but the "lactation risk category" reported by Thomas Hale in his book Medications and Mothers' Milk lists azathioprine as "L3", termed "moderately safe".[66]
Pharmacology
[edit]Pharmacokinetics
[edit]
- XO: xanthine oxidase
- 6-MP: 6-mercaptopurine
- TPMT: thiopurine methyltransferase
- 6-MMP: 6-methylmercaptopurine
- HPRT: hypoxanthine-guanine phosphoribosyltransferase
- TIMP: thioinosine monophosphate, thioinosinic acid
- MeTIMP: methyl-thioinosine monophosphate
- TGTP: thioguanosine triphosphate
- TdGTP: thio-deoxyguanosine triphosphate
Azathioprine is absorbed from the gut to about 88%. Bioavailability varies greatly between individual patients, between 30 and 90%, because the drug is partly inactivated in the liver. Highest blood plasma concentrations, counting not only the drug itself, but also its metabolites, are reached after 1–2 hours, and the average plasma half-life is 26 to 80 minutes for azathioprine and 3–5 hours for drug plus metabolites. 20 to 30% are bound to plasma proteins while circulating in the bloodstream.[12][31][69][70]
Azathioprine is a prodrug, a substance that is not an active drug itself, but is activated in the body. This happens in several steps; at first, it is slowly and almost completely converted to 6-mercaptopurine (6-MP) by reductive cleavage of the thioether (–S–). This is mediated by glutathione and similar compounds in the intestinal wall, the liver, and on red blood cells, without the aid of enzymes. 6-MP is metabolized analogously to natural purines, giving thioguanosine triphosphate (TGTP) and thiodeoxyguanosine triphosphate (TdGTP) via thioinosine monophosphate (TIMP) and several further intermediates. On a second path, the sulfur atom of 6-MP and TIMP is methylated. The end products of azathioprine metabolism are thiouric acid (38%) and various methylated and hydroxylated purines, which are excreted via the urine.[40][69][70]
Mechanism of action
[edit]Azathioprine inhibits purine synthesis. Purines are needed to produce DNA and RNA. By inhibiting purine synthesis, less DNA and RNA are produced for the synthesis of white blood cells, thus causing immunosuppression.
This section may be too technical for most readers to understand. (December 2014) |
Azathioprine is converted within tissues to 6-MP, some of which is converted, in turn, to 6-thioguanine by the addition of an amino group. Both 6-MP and 6-thioguanine are conjugated with ribose, and then phosphorylated to form the nucleotides thioinosinic acid and thioguanylic acid, respectively.[13] These nucleotides masquerade, respectively, as inosinic acid and guanylic acid; the former is the starting point for purine nucleotide biosynthesis, while the latter is one of the building blocks of DNA and RNA.
- The nucleotides are incorporated into newly synthesized (but nonfunctional) DNA, halting replication.
- The nucleotides act to inhibit glutamine-phosphoribosyl pyrophosphate amidotransferase (GPAT), one of the enzymes involved in purine biosynthesis, one of the earlier steps in the synthesis of DNA and RNA. They achieve GPAT inhibition through a form of negative feedback called product inhibition.[71] Because actively replicating cells (such as cancer cells and the T cells and B cells of the immune system) are most active in synthesizing purine, making new DNA, these cells are most strongly affected.[72][12]
- A portion of the nucleotides is additionally phosphorylated to the triphosphate forms. These bind to GTP-binding protein Rac1, blocking synthesis of the protein Bcl-xL, thus sending activated T cells and mononuclear cells into apoptosis (programmed cell death). Increased apoptosis of mononuclear cells is seen in inflammatory bowel disease patients treated with azathioprine.[72]
Chemistry
[edit]Azathioprine is a thiopurine linked to a second heterocycle (an imidazole derivative) via a thioether. It is a pale yellow solid with a slightly bitter taste and a melting point of 238–245 °C. It is practically insoluble in water and only slightly soluble in lipophilic solvents such as chloroform, ethanol, and diethylether. It dissolves in alkaline aqueous solutions, where it hydrolyzes to 6-mercaptopurine.[69]
Azathioprine is synthesized from 5-chloro-1-methyl-4-nitro-1H-imidazole and 6-mercaptopurine in dimethyl sulfoxide.[73] The synthesis of the former starts with an amide from methylamine and diethyl oxalate, which is then cyclized and chlorinated with phosphorus pentachloride;[74] the nitro group is introduced with nitric and sulfuric acid.
History
[edit]Azathioprine was synthesized by George Herbert Hitchings and Gertrude Elion in 1957 (named BW 57-322) to produce 6-MP in a metabolically active, but masked form, and at first used as a chemotherapy drug.[75][76][77]
Robert Schwartz investigated the effect of 6-MP on the immune response in 1958 and discovered that it profoundly suppresses the formation of antibodies when given to rabbits together with antigens.[78] Following the work done by Sir Peter Medawar and Gertrude Elion in discovering the immunological basis of rejection of transplanted tissues and organs, and Schwartz's researches on 6-MP, Sir Roy Calne, the British pioneer in transplantation, introduced 6-MP as an experimental immunosuppressant for kidney and heart transplants.[79] When Calne asked Elion for related compounds to investigate, she suggested azathioprine, which was subsequently found out to be superior (as effective and less toxic to the bone marrow) by Calne.[75][12]
In April 1962, with regimens consisting of azathioprine and prednisone, the transplantation of kidneys to unrelated recipients (allotransplantation) was successful for the first time.[12][80] For many years, this kind of dual therapy with azathioprine and glucocorticoids was the standard antirejection regimen, until cyclosporin was introduced into clinical practice (by Calne as well) in 1978.
Cyclosporin has now replaced some of the azathioprine use due to a longer survival time, especially in heart-related transplantations.[81][82][83] Moreover, despite being considerably more expensive, mycophenolate mofetil is also increasingly being used in place of azathioprine in organ transplantation, as it is associated with less bone marrow suppression, fewer opportunistic infections, and a lower incidence of acute rejection.[16][84]
References
[edit]- ^ "Azathioprine". Merriam-Webster.com Dictionary. Merriam-Webster.
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ "Jayempi EPAR". European Medicines Agency. 20 April 2021. Retrieved 4 March 2023.
- ^ "Jayempi Product information". Union Register of medicinal products. Retrieved 3 March 2023.
- ^ a b c d e f g h i "Azathioprine". The American Society of Health-System Pharmacists. Archived from the original on 20 August 2016. Retrieved 8 December 2016.
- ^ Axelrad JE, Lichtiger S, Yajnik V (May 2016). "Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment". World Journal of Gastroenterology (Review). 22 (20): 4794–4801. doi:10.3748/wjg.v22.i20.4794. PMC 4873872. PMID 27239106.
- ^ a b Singer O, McCune WJ (May 2017). "Update on maintenance therapy for granulomatosis with polyangiitis and microscopic polyangiitis". Current Opinion in Rheumatology. 29 (3): 248–253. doi:10.1097/BOR.0000000000000382. PMID 28306595. S2CID 35805200.
- ^ Jordan N, D'Cruz D (2016). "Current and emerging treatment options in the management of lupus". ImmunoTargets and Therapy. 5: 9–20. doi:10.2147/ITT.S40675. PMC 4970629. PMID 27529058.
- ^ a b c Sami N (2016). Autoimmune Bullous Diseases: Approach and Management. Springer. p. 83. ISBN 9783319267289. Archived from the original on 2016-12-21.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "Azathioprine - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ a b c d e f g Patel AA, Swerlick RA, McCall CO (September 2006). "Azathioprine in dermatology: the past, the present, and the future". Journal of the American Academy of Dermatology. 55 (3): 369–389. doi:10.1016/j.jaad.2005.07.059. PMID 16908341.
- ^ a b Evans WE (April 2004). "Pharmacogenetics of thiopurine S-methyltransferase and thiopurine therapy". Therapeutic Drug Monitoring. 26 (2): 186–191. doi:10.1097/00007691-200404000-00018. PMID 15228163. S2CID 34015182.
- ^ a b c d American Society of Health-System Pharmacists (January 2012). "Azathioprine, Azathioprine Sodium". AHFS Drug Information 2012. American Society of Health-System Pharmacists. ISBN 978-1-58528-267-8.
- ^ Nuyttens JJ, Harper J, Jenrette JM, Turrisi AT (January 2005). "Outcome of radiation therapy for renal transplant rejection refractory to chemical immunosuppression". Radiotherapy and Oncology. 74 (1): 17–19. doi:10.1016/j.radonc.2004.08.011. PMID 15683663.
- ^ a b Remuzzi G, Lesti M, Gotti E, Ganeva M, Dimitrov BD, Ene-Iordache B, et al. (August 2004). "Mycophenolate mofetil versus azathioprine for prevention of acute rejection in renal transplantation (MYSS): a randomised trial". Lancet. 364 (9433): 503–512. doi:10.1016/S0140-6736(04)16808-6. PMID 15302193. S2CID 22033113.
- ^ Suarez-Almazor ME, Spooner C, Belseck E (2000). Suarez-Almazor ME (ed.). "Azathioprine for treating rheumatoid arthritis". The Cochrane Database of Systematic Reviews. 2010 (4): CD001461. doi:10.1002/14651858.CD001461. PMC 8406472. PMID 11034720.
- ^ a b Sandborn WJ (1998). "Azathioprine: state of the art in inflammatory bowel disease". Scandinavian Journal of Gastroenterology. Supplement. 225 (234): 92–99. doi:10.1080/003655298750027290. PMID 9515759.
- ^ Biancone L, Tosti C, Fina D, Fantini M, De Nigris F, Geremia A, et al. (June 2003). "Review article: maintenance treatment of Crohn's disease". Alimentary Pharmacology & Therapeutics. 17 (Suppl 2): 31–37. doi:10.1046/j.1365-2036.17.s2.20.x. PMID 12786610. S2CID 23554085.
- ^ Rutgeerts P (October 2004). "Review article: treatment of perianal fistulizing Crohn's disease". Alimentary Pharmacology & Therapeutics. 20 (Suppl 4): 106–110. doi:10.1111/j.1365-2036.2004.02060.x. PMID 15352905. S2CID 71968695.
- ^ Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD (August 2005). "Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine". Gut. 54 (8): 1121–1125. doi:10.1136/gut.2004.049460. PMC 1774897. PMID 16009685.
- ^ Kirschner BS (October 1998). "Safety of azathioprine and 6-mercaptopurine in pediatric patients with inflammatory bowel disease". Gastroenterology. 115 (4): 813–821. doi:10.1016/S0016-5085(98)70251-3. PMID 9753482.
- ^ Timmer A, Patton PH, Chande N, McDonald JW, MacDonald JK (May 2016). "Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis". The Cochrane Database of Systematic Reviews. 2016 (5): CD000478. doi:10.1002/14651858.CD000478.pub4. PMC 7034525. PMID 27192092.
- ^ Abu-Shakra M, Shoenfeld Y (2001). "Azathioprine therapy for patients with systemic lupus erythematosus". Lupus. 10 (3): 152–153. doi:10.1191/096120301676669495. PMID 11315344. S2CID 71558242.
- ^ Olszewska M, Kolacinska-Strasz Z, Sulej J, Labecka H, Cwikla J, Natorska U, et al. (2007). "Efficacy and safety of cyclophosphamide, azathioprine, and cyclosporine (ciclosporin) as adjuvant drugs in pemphigus vulgaris". American Journal of Clinical Dermatology. 8 (2): 85–92. doi:10.2165/00128071-200708020-00004. PMID 17428113. S2CID 10699017.
- ^ Richman DP, Agius MA (December 2003). "Treatment of autoimmune myasthenia gravis". Neurology. 61 (12): 1652–1661. doi:10.1212/01.wnl.0000098887.24618.a0. PMID 14694025. S2CID 24755812.
- ^ Meggitt SJ, Gray JC, Reynolds NJ (March 2006). "Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema: a double-blind, randomised controlled trial". Lancet. 367 (9513): 839–846. doi:10.1016/S0140-6736(06)68340-2. PMID 16530578. S2CID 1616660.
- ^ Casetta I, Iuliano G, Filippini G (February 2009). "Azathioprine for multiple sclerosis". Journal of Neurology, Neurosurgery, and Psychiatry. 80 (2): 131–2, discussion 132. doi:10.1136/jnnp.2008.144972. PMID 19151017. S2CID 207001537.
- ^ Casetta I, Iuliano G, Filippini G (October 2007). "Azathioprine for multiple sclerosis". The Cochrane Database of Systematic Reviews. 2007 (4): CD003982. doi:10.1002/14651858.CD003982.pub2. PMC 6823213. PMID 17943809.
- ^ Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ (May 2012). "Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis". The New England Journal of Medicine. 366 (21): 1968–1977. doi:10.1056/NEJMoa1113354. PMC 3422642. PMID 22607134.
- ^ a b c d Jasek, W, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 4103–9. ISBN 978-3-85200-181-4.
- ^ Weersma RK, Peters FT, Oostenbrug LE, van den Berg AP, van Haastert M, Ploeg RJ, et al. (October 2004). "Increased incidence of azathioprine-induced pancreatitis in Crohn's disease compared with other diseases". Alimentary Pharmacology & Therapeutics. 20 (8): 843–850. doi:10.1111/j.1365-2036.2004.02197.x. PMID 15479355. S2CID 21873238.
- ^ Pecher D, Zelinkova Z, Lucenicova J, Peppelenbosch M, Dokupilova S, Mikusova V, et al. (November 2020). "Porous graphitic carbon based chromatography hyphenated with mass spectrometry: A new strategy for profiling thiopurine nucleotides in patients with inflammatory bowel diseases". Analytica Chimica Acta. 1137 (1137): 64–73. Bibcode:2020AcAC.1137...64P. doi:10.1016/j.aca.2020.08.064. PMID 33153610. S2CID 225287631.
- ^ a b International Agency for Research on Cancer (IARC) (1987). "Azathioprine". Summaries & Evaluations (suppl. 7): 119. Archived from the original on 2006-06-04.
- ^ Dean L (2012). "Azathioprine Therapy and TPMT Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520349. Bookshelf ID: NBK100661.
- ^ a b Zaza G, Cheok M, Krynetskaia N, Thorn C, Stocco G, Hebert JM, et al. (September 2010). "Thiopurine pathway". Pharmacogenetics and Genomics. 20 (9): 573–574. doi:10.1097/FPC.0b013e328334338f. PMC 3098750. PMID 19952870.
- ^ Stocco G, Pelin M, Franca R, De Iudicibus S, Cuzzoni E, Favretto D, et al. (April 2014). "Pharmacogenetics of azathioprine in inflammatory bowel disease: a role for glutathione-S-transferase?". World Journal of Gastroenterology. 20 (13): 3534–3541. doi:10.3748/wjg.v20.i13.3534. PMC 3974520. PMID 24707136.
- ^ Fujita K, Sasaki Y (August 2007). "Pharmacogenomics in drug-metabolizing enzymes catalyzing anticancer drugs for personalized cancer chemotherapy". Current Drug Metabolism. 8 (6): 554–562. doi:10.2174/138920007781368890. PMID 17691917. Archived from the original on 2013-01-12.
{{cite journal}}: CS1 maint: unfit URL (link) - ^ a b c Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui CH, Yee SW, et al. (March 2011). "Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing". Clinical Pharmacology and Therapeutics. 89 (3). Clinical Pharmacogenetics Implementation Consortium: 387–391. doi:10.1038/clpt.2010.320. PMC 3098761. PMID 21270794.
- ^ a b Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8th ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. pp. 107, 936. ISBN 978-3-8047-1763-3.
- ^ Payne K, Newman W, Fargher E, Tricker K, Bruce IN, Ollier WE (May 2007). "TPMT testing in rheumatology: any better than routine monitoring?". Rheumatology. 46 (5): 727–729. doi:10.1093/rheumatology/kel427. PMID 17255139.
- ^ "Label: Imuran - azathioprine tablet". Archived from the original on 20 October 2014. Retrieved 19 October 2014.
- ^ Wang L, Pelleymounter L, Weinshilboum R, Johnson JA, Hebert JM, Altman RB, et al. (June 2010). "Very important pharmacogene summary: thiopurine S-methyltransferase". Pharmacogenetics and Genomics. 20 (6): 401–405. doi:10.1097/FPC.0b013e3283352860. PMC 3086840. PMID 20154640.
- ^ Yang SK, Hong M, Baek J, Choi H, Zhao W, Jung Y, et al. (September 2014). "A common missense variant in NUDT15 confers susceptibility to thiopurine-induced leukopenia". Nature Genetics. 46 (9): 1017–1020. doi:10.1038/ng.3060. PMC 4999337. PMID 25108385.
- ^ National Toxicology Program (10 June 2011). "Report On Carcinogens – Twelfth Edition – 2011". National Toxicology Program. Archived (PDF) from the original on 16 July 2012. Retrieved June 20, 2012.
- ^ "FDA: Cancer Warnings Required for TNF Blockers". FDA. August 4, 2009. Archived from the original on July 3, 2012. Retrieved June 20, 2012.
- ^ International Agency for Research on Cancer (IARC) (1981). "Azathioprine – 5. Summary of Data Reported and Evaluation". Summaries & Evaluations. 26: 47. Archived from the original on 2006-09-06.
- ^ Gombar VK, Enslein K, Blake BW (May 1993). "Carcinogenicity of azathioprine: an S-AR investigation". Mutation Research. 302 (1): 7–12. doi:10.1016/0165-7992(93)90083-8. PMID 7683109.
- ^ McGovern DP, Jewell DP (August 2005). "Risks and benefits of azathioprine therapy". Gut. 54 (8): 1055–1059. doi:10.1136/gut.2004.053231. PMC 1774869. PMID 16009676.
- ^ "Imuran (azathioprine) Tablets and Injection". FDA. May 2011. Archived from the original on March 2, 2012. Retrieved June 20, 2012.
- ^ "Skin cancer alert for organ drug". BBC Online. BBC News. September 15, 2005. Archived from the original on October 14, 2012. Retrieved June 10, 2012.
- ^ O'Donovan P, Perrett CM, Zhang X, Montaner B, Xu YZ, Harwood CA, et al. (September 2005). "Azathioprine and UVA light generate mutagenic oxidative DNA damage". Science. 309 (5742): 1871–1874. Bibcode:2005Sci...309.1871O. doi:10.1126/science.1114233. PMC 2426755. PMID 16166520.
- ^ Sahasranaman S, Howard D, Roy S (August 2008). "Clinical pharmacology and pharmacogenetics of thiopurines". European Journal of Clinical Pharmacology. 64 (8): 753–767. doi:10.1007/s00228-008-0478-6. PMID 18506437. S2CID 27475772.
- ^ Chocair P, Duley J, Simmonds HA, Cameron JS, Ianhez L, Arap S, et al. (July 1993). "Low-dose allopurinol plus azathioprine/cyclosporin/prednisolone, a novel immunosuppressive regimen". Lancet. 342 (8863): 83–84. doi:10.1016/0140-6736(93)91287-V. PMID 8100914. S2CID 13419507.
- ^ Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D, et al. (September 2005). "Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine". Alimentary Pharmacology & Therapeutics. 22 (5): 441–446. doi:10.1111/j.1365-2036.2005.02583.x. PMID 16128682. S2CID 9356163.
- ^ Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB (February 2007). "Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine". Clinical Gastroenterology and Hepatology. 5 (2): 209–214. doi:10.1016/j.cgh.2006.11.020. PMID 17296529.
- ^ Govani SM, Higgins PD (October 2010). "Combination of thiopurines and allopurinol: adverse events and clinical benefit in IBD". Journal of Crohn's & Colitis. 4 (4): 444–449. doi:10.1016/j.crohns.2010.02.009. PMC 3157326. PMID 21122542.
- ^ Ansari A, Patel N, Sanderson J, O'Donohue J, Duley JA, Florin TH (March 2010). "Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease". Alimentary Pharmacology & Therapeutics. 31 (6): 640–647. doi:10.1111/j.1365-2036.2009.04221.x. PMID 20015102. S2CID 6000856.
- ^ Oliveira A, Sanches M, Selores M (October 2011). "Azathioprine-induced pellagra". The Journal of Dermatology. 38 (10): 1035–1037. doi:10.1111/j.1346-8138.2010.01189.x. PMID 21658113. S2CID 3396280.
- ^ a b Mehta DK, et al. (Pharmaceutical Society of Great Britain) (March 2003). British National Formulary, Issue 45. London: British Medical Association. ISBN 978-0-85369-555-4.
- ^ Cleary BJ, Källén B (July 2009). "Early pregnancy azathioprine use and pregnancy outcomes". Birth Defects Research. Part A, Clinical and Molecular Teratology. 85 (7): 647–654. doi:10.1002/bdra.20583. PMID 19343728.
- ^ Tagatz GE, Simmons RL (January 1975). "Pregnancy after renal transplantation". Annals of Internal Medicine. 82 (1): 113–114. doi:10.7326/0003-4819-82-1-113. PMID 799904.
- ^ Nørgård B, Pedersen L, Fonager K, Rasmussen SN, Sørensen HT (March 2003). "Azathioprine, mercaptopurine and birth outcome: a population-based cohort study". Alimentary Pharmacology & Therapeutics. 17 (6): 827–834. doi:10.1046/j.1365-2036.2003.01537.x. PMID 12641505. S2CID 25314258.
- ^ Tallent MB, Simmons RL, Najarian JS (March 1970). "Birth defects in child of male recipient of kidney transplant". JAMA. 211 (11): 1854–1855. doi:10.1001/jama.211.11.1854. PMID 4905893.
- ^ Polifka JE, Friedman JM (May 2002). "Teratogen update: azathioprine and 6-mercaptopurine". Teratology. 65 (5): 240–261. CiteSeerX 10.1.1.566.7676. doi:10.1002/tera.10043. PMID 11967923.
- ^ Hale TW (April 2010). Medications and Mothers' Milk: A Manual of Lactational Pharmacology. Hale Pub. ISBN 978-0-9823379-9-8.
- ^ Cronstein BN (November 2004). "Pharmacogenetics in the rheumatic diseases". Annals of the Rheumatic Diseases. 63 (Suppl 2): ii25–ii27. doi:10.1136/ard.2004.028217. PMC 1766779. PMID 15479867.
- ^ Karran P, Attard N (January 2008). "Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer". Nature Reviews. Cancer. 8 (1): 24–36. doi:10.1038/nrc2292. PMID 18097462. S2CID 23327335.
- ^ a b c Dinnendahl, V, Fricke, U, eds. (2011). Arzneistoff-Profile (in German). Vol. 2 (25th ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-9846-3.
- ^ a b Steinhilber D, Schubert-Zsilavecz M, Roth HJ (2005). Medizinische Chemie (in German). Stuttgart: Deutscher Apotheker Verlag. p. 340. ISBN 978-3-7692-3483-1.
- ^ "Azathioprine Pathway". Small Molecule Pathway Database. Archived from the original on 2 July 2012. Retrieved 31 August 2012.
- ^ a b Maltzman JS, Koretzky GA (April 2003). "Azathioprine: old drug, new actions". The Journal of Clinical Investigation. 111 (8): 1122–1124. doi:10.1172/JCI18384. PMC 152947. PMID 12697731.
- ^ US Patent 3056785, G. H. Hitchings; Yonkers & G. B. Elion, "Purine Derivatives", issued 1962-10-06.
- ^ Blicke FF, Godt HC (1954). "Diuretics. I. 3-Substituted Paraxanthines". Journal of the American Chemical Society. 76 (14): 3653–3655. doi:10.1021/ja01643a015.
- ^ a b Elion GB (April 1989). "The purine path to chemotherapy". Science. 244 (4900): 41–47. Bibcode:1989Sci...244...41E. doi:10.1126/science.2649979. PMID 2649979.
- ^ Elion GB, Callahan SW, Hitchings GH, Rundles RW (July 1960). "The metabolism of 2-amino-6-[(1-methyl-4-nitro-5-imidazolyl)thio]purine (B.W. 57-323) in man". Cancer Chemotherapy Reports. 8: 47–52. PMID 13849699.
- ^ Thiersch JB (December 1962). "Effect of 6-(1'-methyl-4'-nitro-5'-imidazolyl)-mercaptopurine and 2-amino-6-(1'-methyl-4'-nitro-5'-imidazolyl)-mercaptopurine on the rat litter in utero". Journal of Reproduction and Fertility. 4 (3): 297–302. doi:10.1530/jrf.0.0040297. PMID 13980986.
- ^ Schwartz R, Stack J, Dameshek W (October 1958). "Effect of 6-mercaptopurine on antibody production". Proceedings of the Society for Experimental Biology and Medicine. 99 (1): 164–167. doi:10.3181/00379727-99-24281. PMID 13601801. S2CID 8043359.
- ^ Calne RY (February 1960). "The rejection of renal homografts. Inhibition in dogs by 6-mercaptopurine". Lancet. 1 (7121): 417–418. doi:10.1016/S0140-6736(60)90343-3. PMID 13807024.
- ^ Murray JE, Merrill JP, Harrison JH, Wilson RE, Dammin GJ (June 1963). "Prolonged survival of human-kidney homografts by immunosuppressive drug therapy". The New England Journal of Medicine. 268 (24): 1315–1323. doi:10.1056/NEJM196306132682401. PMID 13936775.
- ^ Bakker RC, Hollander AA, Mallat MJ, Bruijn JA, Paul LC, de Fijter JW (September 2003). "Conversion from cyclosporine to azathioprine at three months reduces the incidence of chronic allograft nephropathy". Kidney International. 64 (3): 1027–1034. doi:10.1046/j.1523-1755.2003.00175.x. PMID 12911553.
- ^ Henry ML, Sommer BG, Ferguson RM (November 1985). "Beneficial effects of cyclosporine compared with azathioprine in cadaveric renal transplantation". American Journal of Surgery. 150 (5): 533–536. doi:10.1016/0002-9610(85)90431-3. PMID 2998215.
- ^ Modry DL, Oyer PE, Jamieson SW, Stinson EB, Baldwin JC, Reitz BA, et al. (May 1985). "Cyclosporine in heart and heart-lung transplantation". Canadian Journal of Surgery. Journal Canadien de Chirurgie. 28 (3): 274–80, 282. PMID 3922606.
- ^ Woodroffe R, Yao GL, Meads C, Bayliss S, Ready A, Raftery J, et al. (May 2005). "Clinical and cost-effectiveness of newer immunosuppressive regimens in renal transplantation: a systematic review and modelling study". Health Technology Assessment. 9 (21): 1–194. doi:10.3310/hta9210. PMID 15899149.
Further reading
[edit]- Dean L (2012). "Azathioprine Therapy and TPMT Genotype". In Pratt VM, McLeod HL, Rubinstein WS, et al. (eds.). Medical Genetics Summaries. National Center for Biotechnology Information (NCBI). PMID 28520349. Bookshelf ID: NBK100661.

