Coenzyme Q10: Difference between revisions
Citation bot (talk | contribs) Add: pmc, pmid, page, doi-access, pages, issue, volume, journal, date, title, authors 1-3. | Use this bot. Report bugs. | Suggested by Zefr | #UCB_toolbar |
|||
| Line 160: | Line 160: | ||
===Bioavailability=== |
===Bioavailability=== |
||
In contrast to intake of CoQ<sub>10</sub> as a constituent of food such as nuts or meat from where CoQ<sub>10</sub> is normally absorbed, there is a concern of CoQ<sub>10</sub> bioavailability when it is used as a dietary supplement.<ref name="a">{{cite journal|doi=10.1177/2156587211399438}}</ref><ref>{{cite journal|doi=10.3390/antiox9050386}}</ref><ref>{{cite journal|doi=10.3390/nu11030527}}</ref>, which is known to be poor due to its lipophilic nature and large molecular weight.<ref name="a"/> In order to find a principle to boost the bioavailability of CoQ<sub>10</sub> after oral administration, several new approaches have been taken; different formulations and forms have been developed and tested on animals and humans.<ref name="Žmitek-2008"/> |
In contrast to intake of CoQ<sub>10</sub> as a constituent of food such as nuts or meat from where CoQ<sub>10</sub> is normally absorbed, there is a concern of CoQ<sub>10</sub> bioavailability when it is used as a dietary supplement.<ref name="a">{{cite journal|doi=10.1177/2156587211399438 |title=Coenzyme Q<sub>10</sub>: Clinical Update and Bioavailability |date=2011 |journal=Journal of Evidence-Based Complementary & Alternative Medicine |volume=16 |issue=2 |pages=129–137 | vauthors = Bank G, Kagan D, Madhavi D }}</ref><ref>{{cite journal|doi=10.3390/antiox9050386|doi-access=free |title=Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism |date=2020 |journal=Antioxidants |volume=9 |issue=5 |page=386 |pmid=32380795 | vauthors = Mantle D, Dybring A }}</ref><ref>{{cite journal|doi=10.3390/nu11030527|doi-access=free |title=Bioavailability and Sustained Plasma Concentrations of CoQ10 in Healthy Volunteers by a Novel Oral Timed-Release Preparation |date=2019 |journal=Nutrients |volume=11 |issue=3 |page=527 |pmid=30823449 |pmc=6471387 | vauthors = Martucci A, Reurean-Pintilei D, Manole A }}</ref>, which is known to be poor due to its lipophilic nature and large molecular weight.<ref name="a"/> In order to find a principle to boost the bioavailability of CoQ<sub>10</sub> after oral administration, several new approaches have been taken; different formulations and forms have been developed and tested on animals and humans.<ref name="Žmitek-2008"/> |
||
====Reduction of particle size==== |
====Reduction of particle size==== |
||
Revision as of 16:28, 16 April 2024

| |
| Names | |
|---|---|
| Preferred IUPAC name
2-[(2E,6E,10E,14E,18E,22E,26E,30E,34E)-3,7,11,15,19,23,27,31,35,39-Decamethyltetraconta-2,6,10,14,18,22,26,30,34,38-decaen-1-yl]-5,6-dimethoxy-3-methylcyclohexa-2,5-diene-1,4-dione | |
Other names
Q10, CoQ10 /ˌkoʊˌkjuːˈtɛn/ | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.590 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C59H90O4 | |
| Molar mass | 863.365 g·mol−1 |
| Appearance | yellow or orange solid |
| Melting point | 48–52 °C (118–126 °F; 321–325 K) |
| insoluble | |
| Pharmacology | |
| C01EB09 (WHO) | |
| Related compounds | |
Related quinones
|
1,4-Benzoquinone Plastoquinone Ubiquinol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
This article needs attention from an expert in biochemistry. See the talk page for details. (April 2024) |
Coenzyme Q10 (CoQ10 /ˌkoʊkjuːˈtɛn/) also known as ubiquinone, is a naturally occurring biochemical cofactor (coenzyme) and an antioxidant produced by the human body.[1][2] It can also be obtained from dietary sources, such as meat, fish, seed oils, vegetables, and dietary supplements.[1] CoQ10 is found in many organisms, including animals and bacteria.
CoQ10 plays a role in mitochondrial oxidative phosphorylation, aiding in the production of adenosine triphosphate (ATP), which is involved in energy transfer within cells.[1] The structure of CoQ10 consists of a benzoquinone moiety and an isoprenoid side chain, with the "10" referring to the number of isoprenyl chemical subunits in its tail.[3][4][5]
Although a ubiquitous molecule in human tissues, CoQ10 is not a dietary nutrient, does not have a recommended intake level, and its use as a supplement is not associated with any health or anti-disease effect.[1][2]
Biological functions
This article needs attention from an expert in biochemistry. See the talk page for details. (April 2024) |
CoQ10 is a component of the mitochondrial electron transport chain (ETC), where it plays a role in oxidative phosphorylation, a process required for the biosynthesis of adenosine triphosphate, the primary energy source of cells.[1][6][7][5]
CoQ10 is a lipophilic molecule that is located in all biological membranes of human body and serves as a component for the synthesis of ATP and is a life-sustaining cofactor for the three complexes (complex I, complex II, and complex III) of the ETC in the mitochondria.[1][4] CoQ10 has a role in the transport of protons across lysosomal membranes to regulate pH in lysosome functions.[1]
The mitochondrial oxidative phosphorylation process takes place in the inner mitochondrial membrane of eukaryotic cells. This membrane is highly folded into structures called cristae, which increase the surface area available for oxidative phosphorylation. CoQ10 plays a role in this process as an essential cofactor of the ETC located in the inner mitochondrial membrane and serves the following functions:[1][6]
- electron transport in the mitochondrial ETC, by shuttling electrons from mitochondrial complexes like nicotinamide adenine dinucleotide (NADH), ubiquinone reductase (complex I), and succinate ubiquinone reductase (complex II), the fatty acids and branched-chain amino acids oxidation (through flavin-linked dehydrogenases) to ubiquinol–cytochrome-c reductase (complex III) of the ETC:[1][6] CoQ10 participates in fatty acid and glucose metabolism by transferring electrons generated from the reduction of fatty acids and glucose to electron acceptors;[8]
- antioxidant activity as a lipid-soluble antioxidant together with vitamin E, scavenging reactive oxygen species and protecting cells against oxidative stress,[6][5] inhibiting the oxidation of proteins and DNA.[9]
CoQ10 also may influence immune response by modulating the expression of genes involved in inflammation.[10][11][12]
Biochemistry
This article needs attention from an expert in biochemistry. See the talk page for details. (April 2024) |
Coenzymes Q is a coenzyme family that is ubiquitous in animals and many Pseudomonadota,[13] a group of gram-negative bacteria. The fact that the coenzyme is ubiquitous gives the origin of its other name, ubiquinone.[14] In humans, the most common form of coenzymes Q is coenzyme Q10, also called CoQ10 (/ˌkoʊkjuːˈtɛn/) or ubiquinone-10.[1]
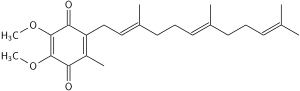
Coenzyme Q10 is a 1,4-benzoquinone, in which "Q" refers to the quinone chemical group and "10" refers to the number of isoprenyl chemical subunits (shown enclosed in brackets in the diagram) in its tail.[1] In natural ubiquinones, there are from six to ten subunits in the tail, with humans having a tail of 10 isoprene units (50 carbon atoms) connected to its benzoquinone "head".[1]
This family of fat-soluble substances is present in all respiring eukaryotic cells, primarily in the mitochondria.[15] Ninety-five percent of the human body's energy is generated this way.[16] Organs with the highest energy requirements—such as the heart, liver, and kidney—have the highest CoQ10 concentrations.[17][18][19][20]
There are three redox states of CoQ: fully oxidized (ubiquinone), semiquinone (ubisemiquinone), and fully reduced (ubiquinol). The capacity of this molecule to act as a two-electron carrier (moving between the quinone and quinol form) and a one-electron carrier (moving between the semiquinone and one of these other forms) is central to its role in the electron transport chain due to the iron–sulfur clusters that can only accept one electron at a time, and as a free radical–scavenging antioxidant.[14][15]
Deficiency
There are two major pathways of deficiency of CoQ10 in humans: reduced biosynthesis, and increased use by the body.[10][21] Biosynthesis is the major source of CoQ10. Biosynthesis requires at least 15 genes, and mutations in any of them can cause CoQ deficiency.[21] CoQ10 levels also may be affected by other genetic defects (such as mutations of mitochondrial DNA, ETFDH, APTX, FXN, and BRAF, genes that are not directly related to the CoQ10 biosynthetic process).[21] Some of these, such as mutations in COQ6, can lead to serious diseases such as steroid-resistant nephrotic syndrome with sensorineural deafness.[22][23][24]
Some adverse effects, largely gastrointestinal, are reported with very high intakes. The observed safe level (OSL) risk assessment method indicated that the evidence of safety is strong at intakes up to 1200 mg/day, and this level is identified as the OSL.[25]
Assessment
Although CoQ10 may be measured in blood plasma, these measurements reflect dietary intake rather than tissue status. Currently, most clinical centers measure CoQ10 levels in cultured skin fibroblasts, muscle biopsies, and blood mononuclear cells.[26] Culture fibroblasts can be used also to evaluate the rate of endogenous CoQ10 biosynthesis, by measuring the uptake of 14C-labeled p-hydroxybenzoate.[27]
Statins
While statins may reduce CoQ10 in the blood it is unclear if they reduce CoQ10 in muscle.[28] Evidence does not support that supplementation improves side effects from statins.[28] However, a more recent metanalysis conducted in China, one of the world's largest producers of this supplement, concluded that, "CoQ10 supplementation ameliorated SAMSs [statin‐associated muscle symptoms], implying that CoQ10 supplementation might be a complementary approach to ameliorate statin‐induced myopathy."[29]
Chemical properties
The oxidized structure of CoQ10 is shown below. The various kinds of coenzyme Q may be distinguished by the number of isoprenoid subunits in their side-chains. The most common coenzyme Q in human mitochondria is CoQ10.[1] Q refers to the quinone head and "10" refers to the number of isoprene repeats in the tail. The molecule below has three isoprenoid units and would be called Q3.
In its pure state, it is an orange-colored lipophile powder, and has no taste nor odor.[14]
Biosynthesis
Biosynthesis occurs in most human tissue. There are three major steps:
- Creation of the benzoquinone structure (using phenylalanine or tyrosine, via 4-hydroxybenzoate)
- Creation of the isoprene side chain (using acetyl-CoA)
- The joining or condensation of the above two structures
The initial two reactions occur in mitochondria, the endoplasmic reticulum, and peroxisomes, indicating multiple sites of synthesis in animal cells.[30]
An important enzyme in this pathway is HMG-CoA reductase, usually a target for intervention in cardiovascular complications. The "statin" family of cholesterol-reducing medications inhibits HMG-CoA reductase. One possible side effect of statins is decreased production of CoQ10, which may be connected to the development of myopathy and rhabdomyolysis. However, the role statins play in CoQ deficiency is controversial. Although statins reduce blood levels of CoQ, studies on the effects of muscle levels of CoQ are yet to come. CoQ supplementation also does not reduce side effects of statin medications.[26][28]
Genes involved include PDSS1, PDSS2, COQ2, and ADCK3 (COQ8, CABC1).[31]
Organisms other than humans produce the benzoquinone and isoprene structures from somewhat different source chemicals. For example, the bacteria E. coli produces the former from chorismate and the latter from a non-mevalonate source. The common yeast S. cerevisiae, however, derives the former from either chorismate or tyrosine and the latter from mevalonate. Most organisms share the common 4-hydroxybenzoate intermediate, yet again uses different steps to arrive at the "Q" structure.[32]
Uses and research
Dietary supplement
Although neither a prescription drug nor an essential nutrient, CoQ10 is commonly used as a dietary supplement with the intent to prevent or improve disease conditions, such as cardiovascular disorders.[14][15][33] CoQ10 is naturally produced by the body and plays a crucial role in cell growth and protection.[5] Despite its significant role in the body, it is not used as a drug for the treatment of any specific disease.[1][2][15]
Nevertheless, CoQ10 is widely available as an over-the-counter dietary supplement and is recommended by some healthcare professionals. Nevertheless, definitive scientific evidence supporting these claims is absent.[1][2][15] Despite the lack of FDA approval, CoQ10 continues to be used in various forms, including in some cosmetics, in spite of the lack of proven benefits.[15]
Regulation and composition
CoQ10 is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of any medical condition.[34][35][36][37] However, it is sold as a dietary supplement in the name of UbiQ 300 & UbiQ 100, not subject to the same regulations as medicinal drugs, and is an ingredient in some cosmetics.[38][39] The manufacture of CoQ10 is not regulated, and different batches and brands may vary significantly:[36] a 2004 laboratory analysis by ConsumerLab.com of CoQ10 supplements on sale in the US found that some did not contain the quantity identified on the product label. Amounts ranged from "no detectable CoQ10", through 75% of stated dose, up to a 75% excess.[40][41]
Generally, CoQ10 is well tolerated. The most common side effects are gastrointestinal symptoms (nausea, vomiting, appetite suppression, and abdominal pain), rashes, and headaches.[42]
Heart disease
A 2014 Cochrane review found insufficient evidence to make a conclusion about its use for the prevention of heart disease.[43] A 2016 Cochrane review concluded that CoQ10 had no effect on blood pressure.[44] A 2021 Cochrane review found "no convincing evidence to support or refute" the use of CoQ10 for the treatment of heart failure.[45]
A 2017 meta-analysis of people with heart failure 30–100 mg/d of CoQ10 found a 31% lower mortality and increased exercise capacity, with no significant difference in the endpoints of left heart ejection fraction.[46]
In a 2023 meta-analysis of older people ubiquinone was compared with ubiquinol. The results demonstrate a beneficial cardiovascular effect of ubiquinone. This could not be confirmed for ubiquinol.[47]
Migraine headaches
The Canadian Headache Society guideline for migraine prophylaxis recommends, based on low-quality evidence, that 300 mg of CoQ10 be offered as a choice for prophylaxis.[48]
Statin myopathy
Although CoQ10 has been used to treat purported muscle-related side effects of statin medications, a 2015 meta-analysis found that CoQ10 had no effect on statin myopathy.[49] A 2018 meta-analysis concluded that there was preliminary evidence for oral CoQ10 reducing statin-associated muscle symptoms, including muscle pain, muscle weakness, muscle cramps and muscle tiredness.[29]
Cancer
As of 2014[update] no large clinical trials of CoQ10 in cancer treatment had been conducted.[36] The US's National Cancer Institute identified issues with the few, small studies that had been carried out, stating, "the way the studies were done and the amount of information reported made it unclear if benefits were caused by the CoQ10 or by something else".[36] The American Cancer Society concluded, "CoQ10 may reduce the effectiveness of chemo and radiation therapy, so most oncologists would recommend avoiding it during cancer treatment."[50]
Dental disease
A 1995 review study found that there is no clinical benefit to the use of CoQ10 in the treatment of periodontal disease.[51]
Pharmacology
Absorption
CoQ10 in the pure form is a crystalline powder insoluble in water. Absorption as a pharmacological substance follows the same process as that of lipids; the uptake mechanism appears to be similar to that of vitamin E, another lipid-soluble nutrient.[20] This process in the human body involves secretion into the small intestine of pancreatic enzymes and bile, which facilitates emulsification and micelle formation required for absorption of lipophilic substances.[52] Food intake (and the presence of lipids) stimulates bodily biliary excretion of bile acids and greatly enhances absorption of CoQ10. Exogenous CoQ10 is absorbed from the small intestine and is best absorbed if taken with a meal. Serum concentration of CoQ10 in fed condition is higher than in fasting conditions.[53][54]
Metabolism
Data on the metabolism of CoQ10 taken as a pharmacological substance in animals and humans are limited.[20] A study with 14C-labeled CoQ10 in rats showed most of the radioactivity in the liver two hours after oral administration when the peak plasma radioactivity was observed.[55] It appears that CoQ10 is metabolized in all tissues, while a major route for its elimination is biliary and fecal excretion; after the withdrawal of CoQ10 supplementation, the levels return to normal within a few days, irrespective of the type of formulation used.[56]
Pharmacokinetics
Some reports have been published on the pharmacokinetics of CoQ10. The plasma peak can be observed 2–6 hours after oral administration when taken as a pharmacological substance; still, this data is inconclusive, because the differences in the duration of onset of plasma peak levels depended on the design of the study. In some studies, a second plasma peak also was observed at approximately 24 hours after administration, probably due to both enterohepatic recycling and redistribution from the liver to circulation.[52] Deuterium-labeled crystalline CoQ10 was used to investigate pharmacokinetics in humans to determine an elimination half-time of 33 hours.[57]
Bioavailability
In contrast to intake of CoQ10 as a constituent of food such as nuts or meat from where CoQ10 is normally absorbed, there is a concern of CoQ10 bioavailability when it is used as a dietary supplement.[58][59][60], which is known to be poor due to its lipophilic nature and large molecular weight.[58] In order to find a principle to boost the bioavailability of CoQ10 after oral administration, several new approaches have been taken; different formulations and forms have been developed and tested on animals and humans.[20]
Reduction of particle size
Nanoparticles have been explored as a delivery system for various drugs, such as improving the oral bioavailability of drugs with poor absorption characteristics.[61] However, this has not proved successful with CoQ10, although reports have differed widely.[62][63] The use of aqueous suspension of finely powdered CoQ10 in pure water also reveals only a minor effect.[56]
Water-solubility
Facilitating drug absorption by increasing its solubility in water is a common pharmaceutical strategy and also has been shown to be successful for CoQ10. Various approaches have been developed to achieve this goal, with many of them producing significantly better results over oil-based softgel capsules in spite of the many attempts to optimize their composition.[20] Examples of such approaches are use of the aqueous dispersion of solid CoQ10 with the polymer tyloxapol,[64] formulations based on various solubilising agents, such as hydrogenated lecithin,[65] and complexation with cyclodextrins; among the latter, the complex with β-cyclodextrin has been found to have highly increased bioavailability[66][67] and also is used in pharmaceutical and food industries for CoQ10-fortification.[20]
Potential drug interactions
CoQ10 taken as a pharmacological substance has potential to inhibit the effects of theophylline as well as the anticoagulant warfarin; CoQ10 may interfere with warfarin's actions by interacting with cytochrome p450 enzymes thereby reducing the INR, a measure of blood clotting.[68] The structure of CoQ10 is similar to that of vitamin K, which competes with and counteracts warfarin's anticoagulation effects. CoQ10 is not recommended in people taking warfarin due to the increased risk of clotting.[42]
Dietary concentrations
Detailed reviews on occurrence of CoQ10 and dietary intake were published in 2010.[69] Besides the endogenous synthesis within organisms, CoQ10 also is supplied to the organism by various foods.[1] Despite the scientific community's great interest in this compound, however, few studies have been performed to determine the contents of CoQ10 in dietary components.[1] CoQ10 concentrations in various foods:[1]
| Food | CoQ10 concentration (mg/kg) | |
|---|---|---|
| Vegetable oils | soybean oil | 54–280 |
| olive oil | 40–160 | |
| grapeseed oil | 64–73 | |
| sunflower oil | 4–15 | |
| canola oil | 64–73 | |
| Beef | heart | 113 |
| liver | 39–50 | |
| muscle | 26–40 | |
| Pork | heart | 12–128 |
| liver | 23–54 | |
| muscle | 14–45 | |
| Chicken | breast | 8–17 |
| thigh | 24–25 | |
| wing | 11 | |
| Fish | sardine | 5–64 |
| mackerel – red flesh | 43–67 | |
| mackerel – white flesh | 11–16 | |
| salmon | 4–8 | |
| tuna | 5 | |
| Nuts | peanut | 27 |
| walnut | 19 | |
| sesame seed | 18–23 | |
| pistachio | 20 | |
| hazelnut | 17 | |
| almond | 5–14 | |
| Vegetables | parsley | 8–26 |
| broccoli | 6–9 | |
| cauliflower | 2–7 | |
| spinach | up to 10 | |
| Chinese cabbage | 2–5 | |
| Fruit | avocado | 10 |
| blackcurrant | 3 | |
| grape | 6–7 | |
| strawberry | 1 | |
| orange | 1–2 | |
| grapefruit | 1 | |
| apple | 1 | |
| banana | 1 | |
Vegetable oils are the richest sources of dietary CoQ10; Meat and fish also are quite rich in CoQ10 levels over 50 mg/kg may be found in beef, pork, and chicken heart and liver. Dairy products are much poorer sources of CoQ10 than animal tissues. Among vegetables, parsley and perilla are the richest CoQ10 sources, but significant differences in their CoQ10 levels may be found in the literature. Broccoli, grapes, and cauliflower are modest sources of CoQ10. Most fruit and berries represent a poor to very poor source of CoQ10, with the exception of avocados, which have a relatively high CoQ10 content.[69][69]
Intake
In the developed world, the estimated daily intake of CoQ10 has been determined at 3–6 mg per day, derived primarily from meat.[69][69]
South Koreans have an estimated average daily CoQ (Q9 + Q10) intake of 11.6 mg/d, derived primarily from kimchi.[70]
Effect of heat and processing
Cooking by frying reduces CoQ10 content by 14–32%.[71]
History
In 1950, a small amount of CoQ10 was isolated from the lining of a horse's gut, a compound initially called substance SA, but later deemed to be quinone found in many animal tissues.[72] In 1957, the same compound was isolated from mitochondrial membranes of beef heart, with research showing that it transported electrons within mitochondria. It was called Q-275 as a quinone.[72][73] The Q-275/substance SA was later renamed ubiquinone as it was a ubiquitous quinone found in all animal tissues.[72] In 1958, its full chemical structure was reported.[72][74] Ubiquinone was later called either mitoquinone or coenzyme Q due to its participation to the mitochondrial electron transport chain.[72] In 1966, a study reported that reduced CoQ6 was an effective antioxidant in cells.[75]
See also
- Idebenone – synthetic analog with reduced oxidant generating properties
- Mitoquinone mesylate – synthetic analog with improved mitochondrial permeability
References
- ^ a b c d e f g h i j k l m n o p q r "Coenzyme Q10". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2018. Retrieved 13 April 2024.
- ^ a b c d "Coenzyme Q10". National Center for Complementary and Integrative Health, US National Institutes of Health. January 2019. Retrieved 13 April 2024.
- ^ Mantle D, Lopez-Lluch G, Hargreaves IP (January 2023). "Coenzyme Q10 Metabolism: A Review of Unresolved Issues". International Journal of Molecular Sciences. 24 (3): 2585. doi:10.3390/ijms24032585. PMC 9916783. PMID 36768907.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ a b Kadian M, Sharma G, Pandita S, Sharma K, Shrivasatava K, Saini N, et al. (2022). "The Impact of Coenzyme Q10 on Neurodegeneration: A Comprehensive Review". Current Pharmacology Reports. 8: 1–19. doi:10.1007/s40495-021-00273-6.
- ^ a b c d Mantle D, Heaton RA, Hargreaves IP (May 2021). "Coenzyme Q10 and Immune Function: An Overview". Antioxidants. 10 (5): 759. doi:10.3390/antiox10050759. PMC 8150987. PMID 34064686.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ a b c d Pradhan N, Singh C, Singh A (November 2021). "Coenzyme Q10 a mitochondrial restorer for various brain disorders". Naunyn Schmiedebergs Arch Pharmacol. 394 (11): 2197–2222. doi:10.1007/s00210-021-02161-8. PMID 34596729.
- ^ Liaghat M, Yaghoubzad-Maleki M, Nabi-Afjadi M, Fathi Z, Zalpoor H, Heidari N, et al. (August 2023). "A Review of the Potential Role of CoQ10 in the Treatment of Hepatocellular Carcinoma". Biochem Genet. doi:10.1007/s10528-023-10490-x. PMID 37632587.
- ^ Manzar H, Abdulhussein D, Yap TE, Cordeiro MF (December 2020). "Cellular Consequences of Coenzyme Q10 Deficiency in Neurodegeneration of the Retina and Brain". Int J Mol Sci. 21 (23): 9299. doi:10.3390/ijms21239299. PMC 7730520. PMID 33291255.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Di Lorenzo A, Iannuzzo G, Parlato A, Cuomo G, Testa C, Coppola M, et al. (April 2020). "Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement". J Clin Med. 9 (5): 1266. doi:10.3390/jcm9051266. PMC 7287951. PMID 32349341.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ a b Hargreaves I, Heaton RA, Mantle D (September 2020). "Disorders of Human Coenzyme Q10 Metabolism: An Overview". Int J Mol Sci. 21 (18): 6695. doi:10.3390/ijms21186695. PMC 7555759. PMID 32933108.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Mantle D, Millichap L, Castro-Marrero J, Hargreaves IP (August 2023). "Primary Coenzyme Q10 Deficiency: An Update". Antioxidants (Basel). 12 (8): 1652. doi:10.3390/antiox12081652. PMC 10451954. PMID 37627647.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Barcelos IP, Haas RH (May 2019). "CoQ10 and Aging". Biology (Basel). 8 (2): 28. doi:10.3390/biology8020028. PMC 6627360. PMID 31083534.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Nowicka B, Kruk J (September 2010). "Occurrence, biosynthesis and function of isoprenoid quinones". Biochimica et Biophysica Acta (BBA) - Bioenergetics. 1797 (9): 1587–1605. doi:10.1016/j.bbabio.2010.06.007. PMID 20599680.
- ^ a b c d
 This article incorporates public domain material from "Ubidecarenone". PubChem. US National Library of Medicine. 30 March 2024. Retrieved 4 April 2024.
This article incorporates public domain material from "Ubidecarenone". PubChem. US National Library of Medicine. 30 March 2024. Retrieved 4 April 2024.
- ^ a b c d e f Sood B, Patel P, Keenaghan M (2024). Coenzyme Q10. PMID 30285386.
- ^ Ernster L, Dallner G (May 1995). "Biochemical, physiological and medical aspects of ubiquinone function". Biochimica et Biophysica Acta - Molecular Basis of Disease. 1271 (1): 195–204. doi:10.1016/0925-4439(95)00028-3. PMID 7599208.
- ^ Okamoto T, Matsuya T, Fukunaga Y, Kishi T, Yamagami T (1989). "Human serum ubiquinol-10 levels and relationship to serum lipids". International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin- und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition. 59 (3): 288–292. PMID 2599795.
- ^ Aberg F, Appelkvist EL, Dallner G, Ernster L (June 1992). "Distribution and redox state of ubiquinones in rat and human tissues". Archives of Biochemistry and Biophysics. 295 (2): 230–234. doi:10.1016/0003-9861(92)90511-T. PMID 1586151.
- ^ Shindo Y, Witt E, Han D, Epstein W, Packer L (January 1994). "Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin". The Journal of Investigative Dermatology. 102 (1): 122–124. doi:10.1111/1523-1747.ep12371744. PMID 8288904.
- ^ a b c d e f Žmitek J, ŽMitek K, Pravs I (2008). "Improving the bioavailability of coenzyme q10 from theory to practice". Agro Food Industry Hi-Tech.
- ^ a b c Desbats MA, Lunardi G, Doimo M, Trevisson E, Salviati L (January 2015). "Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency". J Inherit Metab Dis. 38 (1): 145–56. doi:10.1007/s10545-014-9749-9. PMID 25091424.
- ^ Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, et al. (2011). "COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness". Journal of Clinical Investigation. 121 (5): 2013–2024. doi:10.1172/JCI45693. PMC 3083770. PMID 21540551.
- ^ Justine Perrin R, Rousset-Rouvière C, Garaix F, Cano A, Conrath J, Boyer O, et al. (2020). "COQ6 mutation in patients with nephrotic syndrome, sensorineural deafness, and optic atrophy". Jimd Reports. 54 (1): 37–44. doi:10.1002/jmd2.12068. PMC 7358665. PMID 32685349.
- ^ "Nephrotic Syndrome - COQ6 Associated (Concept Id: C4054393) - MedGen - NCBI".
- ^ Hathcock JN, Shao A (August 2006). "Risk assessment for coenzyme Q10 (Ubiquinone)". Regulatory Toxicology and Pharmacology. 45 (3): 282–288. doi:10.1016/j.yrtph.2006.05.006. PMID 16814438.
- ^ a b Trevisson E, DiMauro S, Navas P, Salviati L (October 2011). "Coenzyme Q deficiency in muscle". Current Opinion in Neurology. 24 (5): 449–456. doi:10.1097/WCO.0b013e32834ab528. hdl:10261/129020. PMID 21844807.
- ^ Montero R, Sánchez-Alcázar JA, Briones P, Hernández AR, Cordero MD, Trevisson E, et al. (June 2008). "Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes". Clinical Biochemistry. 41 (9): 697–700. doi:10.1016/j.clinbiochem.2008.03.007. hdl:11577/2447079. PMID 18387363.
- ^ a b c Tan JT, Barry AR (June 2017). "Coenzyme Q10 supplementation in the management of statin-associated myalgia". American Journal of Health-System Pharmacy. 74 (11): 786–793. doi:10.2146/ajhp160714. PMID 28546301. S2CID 3825396.
- ^ a b Qu H, Guo M, Chai H, Wang WT, Gao ZY, Shi DZ (October 2018). "Effects of Coenzyme Q10 on Statin-Induced Myopathy: An Updated Meta-Analysis of Randomized Controlled Trials". Journal of the American Heart Association. 7 (19): e009835. doi:10.1161/JAHA.118.009835. PMC 6404871. PMID 30371340.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Bentinger M, Tekle M, Dallner G (May 2010). "Coenzyme Q--biosynthesis and functions". Biochemical and Biophysical Research Communications. 396 (1): 74–79. doi:10.1016/j.bbrc.2010.02.147. PMID 20494114.
- ^ Espinós C, Felipo V, Palau F (2009). Inherited Neuromuscular Diseases: Translation from Pathomechanisms to Therapies. Springer. pp. 122ff. ISBN 978-90-481-2812-9. Retrieved 4 January 2011.
- ^ Meganathan R (September 2001). "Ubiquinone biosynthesis in microorganisms". FEMS Microbiology Letters. 203 (2): 131–139. doi:10.1111/j.1574-6968.2001.tb10831.x. PMID 11583838.
- ^ Arenas-Jal M, Suñé-Negre JM, García-Montoya E (March 2020). "Coenzyme Q10 supplementation: Efficacy, safety, and formulation challenges". Comprehensive Reviews in Food Science and Food Safety. 19 (2): 574–594. doi:10.1111/1541-4337.12539. hdl:2445/181270. PMID 33325173.
- ^
 This article incorporates public domain material from Coenzyme Q10. National Cancer Institute. April 2022.
This article incorporates public domain material from Coenzyme Q10. National Cancer Institute. April 2022.
- ^ PDQ Integrative, Alternative, and Complementary Therapies Editorial Board (2002). Coenzyme Q10: Health Professional Version. PDQ Integrative, Alternative, and Complementary Therapies Editorial Board. PMID 26389329.
- ^ a b c d
 This article incorporates public domain material from White J (14 May 2014). PDQ Coenzyme Q10. National Cancer Institute, National Institutes of Health, U.S. Dept. of Health and Human Services. Retrieved 29 June 2014.
This article incorporates public domain material from White J (14 May 2014). PDQ Coenzyme Q10. National Cancer Institute, National Institutes of Health, U.S. Dept. of Health and Human Services. Retrieved 29 June 2014.
- ^ "Mitochondrial disorders in children: Co-enzyme Q10". nice.org.uk. UK: National Institute for Health and Care Excellence. 28 March 2017. Archived from the original on 10 October 2019. Retrieved 10 October 2019.
- ^ Hojerová J (May 2000). "[Coenzyme Q10--its importance, properties and use in nutrition and cosmetics]". Ceska a Slovenska Farmacie. 49 (3): 119–123. PMID 10953455.
- ^ Gardner SS (ed.). 10-and-why-is-it-in-skin-care-products "What is coenzyme Q10 (CoQ10) and why is it in skin care products?". WebMD. 10-and-why-is-it-in-skin-care-products Archived from the original on 13 April 2020. Retrieved 3 May 2020.
- ^ "ConsumerLab.com finds discrepancies in strength of CoQ10 supplements". Townsend Letter for Doctors and Patients. August–September 2004. p. 19.
- ^ 10-coenzyme-q10-tests/01-13-2004/ "ConsumerLab.com finds discrepancies in strength of CoQ10 supplements". ConsumerLab.com. January 2004. 10-coenzyme-q10-tests/01-13-2004/ Archived from the original on 11 November 2021. Retrieved 11 November 2021.
- ^ a b Wyman M, Leonard M, Morledge T (July 2010). "Coenzyme Q10: a therapy for hypertension and statin-induced myalgia?". Cleveland Clinic Journal of Medicine. 77 (7): 435–442. doi:10.3949/ccjm.77a.09078. PMID 20601617. S2CID 26572524.
- ^ Flowers N, Hartley L, Todkill D, Stranges S, Rees K (4 December 2014). "Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease". The Cochrane Database of Systematic Reviews. 2014 (12): CD010405. doi:10.1002/14651858.CD010405.pub2. PMC 9759150. PMID 25474484.
- ^ Ho MJ, Li EC, Wright JM (March 2016). "Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension". The Cochrane Database of Systematic Reviews. 2016 (3): CD007435. doi:10.1002/14651858.CD007435.pub3. PMC 6486033. PMID 26935713.
- ^ Al Saadi T, Assaf Y, Farwati M, Turkmani K, Al-Mouakeh A, Shebli B, et al. (Cochrane Heart Group) (February 2021). "Coenzyme Q10 for heart failure". The Cochrane Database of Systematic Reviews. 2021 (2): CD008684. doi:10.1002/14651858.CD008684.pub3. PMC 8092430. PMID 35608922.
- ^ Lei L, Liu Y (July 2017). "Efficacy of coenzyme Q10 in patients with cardiac failure: a meta-analysis of clinical trials". BMC Cardiovascular Disorders. 17 (1): 196. doi:10.1186/s12872-017-0628-9. PMC 5525208. PMID 28738783.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Fladerer JP, Grollitsch S (December 2023). "Comparison of Coenzyme Q10 (Ubiquinone) and Reduced Coenzyme Q10 (Ubiquinol) as Supplement to Prevent Cardiovascular Disease and Reduce Cardiovascular Mortality". Current Cardiology Reports. 25 (12): 1759–1767. doi:10.1007/s11886-023-01992-6. PMC 10811087. PMID 37971634.
- ^ Pringsheim T, Davenport W, Mackie G, Worthington I, Aubé M, Christie SN, et al. (March 2012). "Canadian Headache Society guideline for migraine prophylaxis". The Canadian Journal of Neurological Sciences. Le Journal Canadien des Sciences Neurologiques. 39 (2 Suppl 2): S1-59. PMID 22683887.
- ^ Banach M, Serban C, Sahebkar A, Ursoniu S, Rysz J, Muntner P, et al. (January 2015). "Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials". Mayo Clinic Proceedings (Systematic Review and Meta-Analysis). 90 (1): 24–34. doi:10.1016/j.mayocp.2014.08.021. PMID 25440725.
- ^ "Coenzyme Q10". American Cancer Society. Archived from the original on 24 February 2014. Retrieved 20 February 2014.
- ^ Watts TL (March 1995). "Coenzyme Q10 and periodontal treatment: is there any beneficial effect?". British Dental Journal. 178 (6): 209–213. doi:10.1038/sj.bdj.4808715. PMID 7718355. S2CID 7207070.
- ^ a b Bhagavan HN, Chopra RK (May 2006). "Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics". Free Radical Research. 40 (5): 445–453. doi:10.1080/10715760600617843. PMID 16551570. S2CID 39001523.
- ^ Bogentoft C, Edlund PO, Olsson B, Widlund L, Westensen K (1991). "Biopharmaceutical aspects of intravenous and oral administration of coenzyme Q10.". Biomedical and clinical aspects of coenzyme Q. Vol. 6. pp. 215–224.
- ^ Ochiai A, Itagaki S, Kurokawa T, Kobayashi M, Hirano T, Iseki K (August 2007). "Improvement in intestinal coenzyme q10 absorption by food intake". Yakugaku Zasshi. 127 (8): 1251–1254. doi:10.1248/yakushi.127.1251. hdl:2115/30144. PMID 17666877.[verification needed]
- ^ Kishi H, Kanamori N, Nisii S, Hiraoka E, Okamoto T, Kishi T (1964). "Metabolism and Exogenous Coenzyme Q10 in vivo and Bioavailability of Coenzyme Q10 Preparations in Japan". Biomedical and Clinical Aspects of Coenzyme Q. Amsterdam: Elsevier. pp. 131–142.
- ^ a b Ozawa Y, Mizushima Y, Koyama I, Akimoto M, Yamagata Y, Hayashi H, et al. (April 1986). "Intestinal absorption enhancement of coenzyme Q10 with a lipid microsphere". Arzneimittel-Forschung. 36 (4): 689–690. PMID 3718593.
- ^ Tomono Y, Hasegawa J, Seki T, Motegi K, Morishita N (October 1986). "Pharmacokinetic study of deuterium-labeled coenzyme Q10 in man". International Journal of Clinical Pharmacology, Therapy, and Toxicology. 24 (10): 536–541. PMID 3781673.
- ^ a b Bank G, Kagan D, Madhavi D (2011). "Coenzyme Q10: Clinical Update and Bioavailability". Journal of Evidence-Based Complementary & Alternative Medicine. 16 (2): 129–137. doi:10.1177/2156587211399438.
- ^ Mantle D, Dybring A (2020). "Bioavailability of Coenzyme Q10: An Overview of the Absorption Process and Subsequent Metabolism". Antioxidants. 9 (5): 386. doi:10.3390/antiox9050386. PMID 32380795.
- ^ Martucci A, Reurean-Pintilei D, Manole A (2019). "Bioavailability and Sustained Plasma Concentrations of CoQ10 in Healthy Volunteers by a Novel Oral Timed-Release Preparation". Nutrients. 11 (3): 527. doi:10.3390/nu11030527. PMC 6471387. PMID 30823449.
- ^ Mathiowitz E, Jacob JS, Jong YS, Carino GP, Chickering DE, Chaturvedi P, et al. (March 1997). "Biologically erodable microspheres as potential oral drug delivery systems". Nature. 386 (6623): 410–414. Bibcode:1997Natur.386..410M. doi:10.1038/386410a0. PMID 9121559. S2CID 4324209.
- ^ Hsu CH, Cui Z, Mumper RJ, Jay M (2003). "Preparation and characterization of novel coenzyme Q10 nanoparticles engineered from microemulsion precursors". AAPS PharmSciTech. 4 (3): E32. doi:10.1208/pt040332. PMC 2750625. PMID 14621964.[verification needed]
- ^ Joshi SS, Sawant SV, Shedge A, Halpner AD (January 2003). "Comparative bioavailability of two novel coenzyme Q10 preparations in humans". International Journal of Clinical Pharmacology and Therapeutics. 41 (1): 42–48. doi:10.5414/CPP41042. PMID 12564745.[verification needed]
- ^ US 6197349, Westesen K, Siekmann B, "Particles with modified physicochemical properties, their preparation and uses", published 2001
- ^ US 4483873, Ohashi H, Takami T, Koyama N, Kogure Y, Ida K, "Aqueous solution containing ubidecarenone", published 1984
- ^ Zmitek J, Smidovnik A, Fir M, Prosek M, Zmitek K, Walczak J, et al. (2008). "Relative bioavailability of two forms of a novel water-soluble coenzyme Q10". Annals of Nutrition & Metabolism. 52 (4): 281–287. doi:10.1159/000129661. PMID 18645245. S2CID 825159.
- ^ Kagan D, Madhavi D (2010). "A Study on the Bioavailability of a Novel Sustained-Release Coenzyme Q10-β-Cyclodextrin Complex". Integrative Medicine. 9 (1).
- ^ Sharma A, Fonarow GC, Butler J, Ezekowitz JA, Felker GM (April 2016). "Coenzyme Q10 and Heart Failure: A State-of-the-Art Review". Circulation. Heart Failure. 9 (4): e002639. doi:10.1161/CIRCHEARTFAILURE.115.002639. PMID 27012265. S2CID 2034503.
- ^ a b c d e f Pravst I, Zmitek K, Zmitek J (April 2010). "Coenzyme Q10 contents in foods and fortification strategies". Critical Reviews in Food Science and Nutrition. 50 (4): 269–280. doi:10.1080/10408390902773037. PMID 20301015. S2CID 38779392.
- ^ Pyo Y, Oh H (2011). "Ubiquinone contents in Korean fermented foods and average daily intakes". Journal of Food Composition and Analysis. 24 (8): 1123–1129. doi:10.1016/j.jfca.2011.03.018.
- ^ Weber C, Bysted A, Hłlmer G (1997). "The coenzyme Q10 content of the average Danish diet". International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin- und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition. 67 (2): 123–129. PMID 9129255.
- ^ a b c d e Morton RA (December 1958). "Ubiquinone". Nature. 182 (4652): 1764–1767. Bibcode:1958Natur.182.1764M. doi:10.1038/1821764a0. PMID 13622652.
- ^ Crane FL, Hatefi Y, Lester RL, Widmer C (July 1957). "Isolation of a quinone from beef heart mitochondria". Biochimica et Biophysica Acta. 25 (1): 220–221. doi:10.1016/0006-3002(57)90457-2. PMID 13445756.
- ^ Wolf DE (1958). "Coenzyme Q. I. structure studies on the coenzyme Q group". Journal of the American Chemical Society. 80 (17): 4752. doi:10.1021/ja01550a096. ISSN 0002-7863.
- ^ Mellors A, Tappel AL (July 1966). "Quinones and quinols as inhibitors of lipid peroxidation". Lipids. 1 (4): 282–284. doi:10.1007/BF02531617. PMID 17805631. S2CID 2129339.
External links
- "List of USP Verified CoQ10 Ingredients". U.S. Pharmacopeial Convention. Archived from the original on 9 February 2009.
- Bonakdar RA, Guarneri E (September 2005). "Coenzyme Q10". American Family Physician. 72 (6): 1065–1070. PMID 16190504. Archived from the original on 24 July 2008. Retrieved 13 November 2008.