Autism: Difference between revisions
Tags: Mobile edit Mobile web edit |
Added references for Chd8 gene which was previously mentiones in this page. |
||
| Line 151: | Line 151: | ||
{{as of|2018}}, it appeared that between 74% and 93% of ASD risk is heritable.<ref name="Lord 2018" /> After an older child is diagnosed with ASD, 7–20% of subsequent children are likely to be as well.<ref name="Lord 2018" /> If parents have one autistic child, they have a 2% to 8% chance of having a second child who is also autistic. If the autistic child is an identical twin the other will be affected 36 to 95 percent of the time. If they are fraternal twins the other will only be affected up to 31 percent of the time.{{medcn|date=April 2022}} |
{{as of|2018}}, it appeared that between 74% and 93% of ASD risk is heritable.<ref name="Lord 2018" /> After an older child is diagnosed with ASD, 7–20% of subsequent children are likely to be as well.<ref name="Lord 2018" /> If parents have one autistic child, they have a 2% to 8% chance of having a second child who is also autistic. If the autistic child is an identical twin the other will be affected 36 to 95 percent of the time. If they are fraternal twins the other will only be affected up to 31 percent of the time.{{medcn|date=April 2022}} |
||
{{as of|2018}}, understanding of genetic risk factors had shifted from a focus on a few [[allele]]s to an understanding that genetic involvement in ASD is probably diffuse, depending on a large number of variants, some of which are common and have a small effect, and some of which are rare and have a large effect. The most common gene disrupted with large effect rare variants appeared to be ''[[CHD8]]'', but less than 0.5% of autistic people have such a mutation. The gene CHD8 encodes the protein chromodomain helicase DNA binding protein 8, which is a [[chromatin]] regulator enzyme that is essential during fetal development, CHD8 is an ATP dependent enzyme<ref>{{Cite journal |last=Nishiyama |first=Masaaki |last2=Oshikawa |first2=Kiyotaka |last3=Tsukada |first3=Yu-ichi |last4=Nakagawa |first4=Tadashi |last5=Iemura |first5=Shun-ichiro |last6=Natsume |first6=Tohru |last7=Fan |first7=Yuhong |last8=Kikuchi |first8=Akira |last9=Skoultchi |first9=Arthur I. |last10=Nakayama |first10=Keiichi I. |date=2009-01-18 |title=CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis |url=http://dx.doi.org/10.1038/ncb1831 |journal=Nature Cell Biology |volume=11 |issue=2 |pages=172–182 |doi=10.1038/ncb1831 |issn=1465-7392}}</ref><ref>{{Cite journal |last=Ronan |first=Jehnna L. |last2=Wu |first2=Wei |last3=Crabtree |first3=Gerald R. |date=2013-04-09 |title=From neural development to cognition: unexpected roles for chromatin |url=http://dx.doi.org/10.1038/nrg3413 |journal=Nature Reviews Genetics |volume=14 |issue=5 |pages=347–359 |doi=10.1038/nrg3413 |issn=1471-0056}}</ref><ref>{{Cite journal |last=Thompson |first=Brandi A. |last2=Tremblay |first2=Véronique |last3=Lin |first3=Grace |last4=Bochar |first4=Daniel A. |date=2008-06-15 |title=CHD8 Is an ATP-Dependent Chromatin Remodeling Factor That Regulates β-Catenin Target Genes |url=http://dx.doi.org/10.1128/mcb.00322-08 |journal=Molecular and Cellular Biology |volume=28 |issue=12 |pages=3894–3904 |doi=10.1128/mcb.00322-08 |issn=0270-7306}}</ref>. The protein contains an Snf2 helicase domain that is responsible for the hydrolysis of ATP to ADP<ref>{{Cite journal |last=Thompson |first=Brandi A. |last2=Tremblay |first2=Véronique |last3=Lin |first3=Grace |last4=Bochar |first4=Daniel A. |date=2008-06-15 |title=CHD8 Is an ATP-Dependent Chromatin Remodeling Factor That Regulates β-Catenin Target Genes |url=http://dx.doi.org/10.1128/mcb.00322-08 |journal=Molecular and Cellular Biology |volume=28 |issue=12 |pages=3894–3904 |doi=10.1128/mcb.00322-08 |issn=0270-7306}}</ref>. CHD8 encodes for a DNA helicase that function as a transcription repressor by remodeling chromatin structure by altering the position of nucleosomes. CHD8 negatively regulates Wnt signaling. Wnt signaling is important in the vertebrate early development and morphogenesis. It is believed that CHD8 also recruits the linker histone H1 and causes the repression of β-catenin and p53 target genes<ref>{{Cite journal |last=Nishiyama |first=Masaaki |last2=Oshikawa |first2=Kiyotaka |last3=Tsukada |first3=Yu-ichi |last4=Nakagawa |first4=Tadashi |last5=Iemura |first5=Shun-ichiro |last6=Natsume |first6=Tohru |last7=Fan |first7=Yuhong |last8=Kikuchi |first8=Akira |last9=Skoultchi |first9=Arthur I. |last10=Nakayama |first10=Keiichi I. |date=2009-01-18 |title=CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis |url=http://dx.doi.org/10.1038/ncb1831 |journal=Nature Cell Biology |volume=11 |issue=2 |pages=172–182 |doi=10.1038/ncb1831 |issn=1465-7392}}</ref>. The importance of CHD8 can be observed in studies where CHD8-knockout mice died after 5.5 embryonic days because of widespread p53 induced apoptosis. Some studies have determined the role of CHD8 in autism spectrum disorder (ASD). CHD8 expression significantly increases during human mid-fetal development<ref>{{Cite journal |last=Nishiyama |first=Masaaki |last2=Oshikawa |first2=Kiyotaka |last3=Tsukada |first3=Yu-ichi |last4=Nakagawa |first4=Tadashi |last5=Iemura |first5=Shun-ichiro |last6=Natsume |first6=Tohru |last7=Fan |first7=Yuhong |last8=Kikuchi |first8=Akira |last9=Skoultchi |first9=Arthur I. |last10=Nakayama |first10=Keiichi I. |date=2009-01-18 |title=CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis |url=http://dx.doi.org/10.1038/ncb1831 |journal=Nature Cell Biology |volume=11 |issue=2 |pages=172–182 |doi=10.1038/ncb1831 |issn=1465-7392}}</ref>. The chromatin remodeling activity and its interaction with transcriptional regulators have shown to play an important role in ASD aetiology.<ref>{{Cite journal |last=Ronan |first=Jehnna L. |last2=Wu |first2=Wei |last3=Crabtree |first3=Gerald R. |date=2013-04-09 |title=From neural development to cognition: unexpected roles for chromatin |url=http://dx.doi.org/10.1038/nrg3413 |journal=Nature Reviews Genetics |volume=14 |issue=5 |pages=347–359 |doi=10.1038/nrg3413 |issn=1471-0056}}</ref> The developing mammalian brain has a conserved CHD8 target regions that are associated with ASD risk genes<ref>{{Cite journal |last=Sugathan |first=Aarathi |last2=Biagioli |first2=Marta |last3=Golzio |first3=Christelle |last4=Erdin |first4=Serkan |last5=Blumenthal |first5=Ian |last6=Manavalan |first6=Poornima |last7=Ragavendran |first7=Ashok |last8=Brand |first8=Harrison |last9=Lucente |first9=Diane |last10=Miles |first10=Judith |last11=Sheridan |first11=Steven D. |date=2014-10-07 |title=<i>CHD8</i> |
|||
| ⚫ | |||
regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors |url=http://dx.doi.org/10.1073/pnas.1405266111 |journal=Proceedings of the National Academy of Sciences |volume=111 |issue=42 |doi=10.1073/pnas.1405266111 |issn=0027-8424}}</ref>. The knockdown of CHD8 in human neural stem cells results in dysregulation of ASD risk genes that are targeted by CHD8<ref>{{Cite journal |last=Cotney |first=Justin |last2=Muhle |first2=Rebecca A. |last3=Sanders |first3=Stephan J. |last4=Liu |first4=Li |last5=Willsey |first5=A. Jeremy |last6=Niu |first6=Wei |last7=Liu |first7=Wenzhong |last8=Klei |first8=Lambertus |last9=Lei |first9=Jing |last10=Yin |first10=Jun |last11=Reilly |first11=Steven K. |date=2015-03-10 |title=The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment |url=http://dx.doi.org/10.1038/ncomms7404 |journal=Nature Communications |volume=6 |issue=1 |doi=10.1038/ncomms7404 |issn=2041-1723}}</ref>. Recently CD8 has been asscociated to the regulation of long non-coding RNAs (lncRNAs)<ref>{{Cite journal |last=Wilkinson |first=B |last2=Grepo |first2=N |last3=Thompson |first3=B L |last4=Kim |first4=J |last5=Wang |first5=K |last6=Evgrafov |first6=O V |last7=Lu |first7=W |last8=Knowles |first8=J A |last9=Campbell |first9=D B |date=2015-05 |title=The autism-associated gene chromodomain helicase DNA-binding protein 8 (CHD8) regulates noncoding RNAs and autism-related genes |url=http://dx.doi.org/10.1038/tp.2015.62 |journal=Translational Psychiatry |volume=5 |issue=5 |pages=e568–e568 |doi=10.1038/tp.2015.62 |issn=2158-3188}}</ref>, and the regulation of X chromosome inactivation (XCI) initiation, via regulation of Xist long non-coding RNA, the master regulator of XCI, though competitive binding to Xist regulatory regions<ref>{{Cite journal |last=Cerase |first=Andrea |last2=Young |first2=Alexander N. |last3=Ruiz |first3=Nerea Blanes |last4=Buness |first4=Andreas |last5=Sant |first5=Gabrielle M. |last6=Arnold |first6=Mirjam |last7=Di Giacomo |first7=Monica |last8=Ascolani |first8=Michela |last9=Kumar |first9=Manish |last10=Hierholzer |first10=Andreas |last11=Trigiante |first11=Giuseppe |date=2021-04-15 |title=Chd8 regulates X chromosome inactivation in mouse through fine-tuning control of Xist expression |url=http://dx.doi.org/10.1038/s42003-021-01945-1 |journal=Communications Biology |volume=4 |issue=1 |doi=10.1038/s42003-021-01945-1 |issn=2399-3642}}</ref>. |
|||
| ⚫ | Some ASD is associated with clearly genetic conditions, like [[fragile X syndrome]]; however, only around 2% of autistic people have fragile X.<ref name="Lord 2018" /> Hypotheses from [[evolutionary psychiatry]] suggest that these genes persist because they are linked to human inventiveness, intelligence or systemising.<ref>{{cite journal | vauthors = Crespi BJ | title = Autism As a Disorder of High Intelligence | journal = Frontiers in Neuroscience | volume = 10 | pages = 300 | date = 30 June 2016 | pmid = 27445671 | pmc = 4927579 | doi = 10.3389/fnins.2016.00300 | doi-access = free }}</ref><ref>{{Cite book| vauthors = Baron-Cohen S |title=The pattern seekers: how autism drives human invention|date=10 November 2020|isbn=978-1-5416-4713-8|oclc=1204602315}}</ref> |
||
Current research suggests that genes that increase susceptibility to ASD are ones that control [[Protein biosynthesis|protein synthesis]] in [[Neuron|neuronal cells]] in response to cell needs, activity and adhesion of neuronal cells, [[synapse]] formation and remodeling, and excitatory to inhibitory [[neurotransmitter]] balance. Therefore, despite up to 1000 different genes thought to contribute to increased risk of ASD, all of them eventually affect normal neural development and connectivity between different functional areas of the brain in a similar manner that is characteristic of an ASD brain. Some of these genes are known to modulate production of the [[Gamma-Aminobutyric acid|GABA]] neurotransmitter which is the main inhibitory neurotransmitter in the nervous system. These GABA-related genes are underexpressed in an ASD brain. On the other hand, genes controlling expression of [[glia]]l and immune cells in the brain e.g. [[astrocyte]]s and [[microglia]], respectively, are overexpressed which correlates with increased number of glial and immune cells found in postmortem ASD brains. Some genes under investigation in ASD pathophysiology are those that affect the [[mTOR]] signaling pathway which supports cell growth and survival.<ref name=Chen2015>{{cite journal |vauthors=Chen JA, Peñagarikano O, Belgard TG, Swarup V, Geschwind DH |title=The emerging picture of autism spectrum disorder: genetics and pathology |journal=Annu Rev Pathol |volume=10 |pages=111–44 |date=2015 |pmid=25621659 |doi=10.1146/annurev-pathol-012414-040405 |type= Review}}</ref> |
Current research suggests that genes that increase susceptibility to ASD are ones that control [[Protein biosynthesis|protein synthesis]] in [[Neuron|neuronal cells]] in response to cell needs, activity and adhesion of neuronal cells, [[synapse]] formation and remodeling, and excitatory to inhibitory [[neurotransmitter]] balance. Therefore, despite up to 1000 different genes thought to contribute to increased risk of ASD, all of them eventually affect normal neural development and connectivity between different functional areas of the brain in a similar manner that is characteristic of an ASD brain. Some of these genes are known to modulate production of the [[Gamma-Aminobutyric acid|GABA]] neurotransmitter which is the main inhibitory neurotransmitter in the nervous system. These GABA-related genes are underexpressed in an ASD brain. On the other hand, genes controlling expression of [[glia]]l and immune cells in the brain e.g. [[astrocyte]]s and [[microglia]], respectively, are overexpressed which correlates with increased number of glial and immune cells found in postmortem ASD brains. Some genes under investigation in ASD pathophysiology are those that affect the [[mTOR]] signaling pathway which supports cell growth and survival.<ref name=Chen2015>{{cite journal |vauthors=Chen JA, Peñagarikano O, Belgard TG, Swarup V, Geschwind DH |title=The emerging picture of autism spectrum disorder: genetics and pathology |journal=Annu Rev Pathol |volume=10 |pages=111–44 |date=2015 |pmid=25621659 |doi=10.1146/annurev-pathol-012414-040405 |type= Review}}</ref> |
||
Revision as of 13:28, 13 May 2022
This page is currently being merged. After a discussion, consensus to merge this page with autism was found. You can help implement the merge by following the instructions at Help:Merging and the resolution on the discussion. Process started in February 2022. |
| Autism spectrum disorder | |
|---|---|
| Other names |
|
 | |
| Repetitively stacking or lining up objects is associated with autism spectrum disorder. | |
| Specialty | Clinical psychology, psychiatry, pediatrics, occupational medicine |
| Symptoms | Impairments in social interaction, verbal and nonverbal communication, and the presence of restricted interests and repetitive behavior |
| Complications | Social isolation, educational and employment problems,[1] anxiety,[1] stress,[1] bullying,[1] self-harm |
| Onset | Early childhood |
| Duration | Lifelong |
| Causes | Uncertain; multi-factorial |
| Risk factors | Family history, certain genetic conditions, having older parents, certain prescribed drugs, perinatal and neonatal health issues |
| Diagnostic method | Based on observation of behavior and development |
| Differential diagnosis | Intellectual disability, anxiety, depression, Rett syndrome, attention deficit hyperactivity disorder, selective mutism, schizophrenia |
| Management | Speech therapy, Occupational therapy, Behavioral therapy, psychotropic medication |
| Frequency |
|
The autism spectrum is a range of neurodevelopmental conditions primarily characterized by significant difficulties in social interactions, differences in communication, and presentations of rigid and repetitive behavior. Unusual responses to sensory input, including high or low sensitivity, sensory discrimination, and sensory-based motor impairments are also highly prevalent. It is commonly referred to as autism and officially designated autism spectrum disorder (ASD).
A spectrum disorder is one that can manifest very differently from person to person: any given person is likely to show some, but not all of the characteristics associated with it, and may show them to very different degrees. Different autistic people might show strikingly different characteristics, and the same person may also present differently at different times.[2] The autism spectrum was historically divided into sub-categories, but there were persistent questions over the validity of these divisions,[2][3] and the most recent editions of the diagnostic manuals, Diagnostic and Statistical Manual of Mental Disorders (DSM-5, published in 2013) and International Classification of Diseases (ICD-11, released in 2021[4]) both list ASD as a single disorder.[5][6]
There is some dispute over the appropriateness of the term 'disorder',[7][8] and many sources prefer to use the terms autism,[9] or autism spectrum conditions (ASC),[10][11] rather than ASD. While psychiatry traditionally classifies autism as a neurodevelopmental disorder, certain advocates see autism as part of neurodiversity, the natural diversity in human thinking and experience.[12] On this view, promoted by the autism rights movement, there is not necessarily anything wrong with an autistic person, but this does not preclude them being disabled and potentially having high support needs.[13] This relatively positive view of autism has led to a certain degree of friction between autistic individuals, advocates and charities.[14][15]
Other controversies in autism include scientific ones, political and philosophical ones, and many which have aspects of all three. Scientists are still trying to determine what causes autism; it is highly heritable and believed to be mainly genetic, but there are many genes involved, and environmental factors may also be relevant.[16] It is unclear why autism commonly co-occurs with ADHD, intellectual disabilities, epilepsy and a range of other conditions. There are ongoing disagreements about what should be included as part of the autism spectrum, whether meaningful sub-types of autism exist,[17] and the significance of autism-associated traits in the wider population.[18][19] The combination of broader criteria and increased awareness has led to a trend of steadily increasing estimates of autism prevalence, causing a common misconception that there is an 'autism epidemic'[20] and feeding the myth that this is caused by vaccines.
Classification
ICD
The World Health Organization's International Classification of Diseases (11th Revision) ICD-11, regarded as the global standard, was released in June 2018 and came into full effect from January 2022.[21] It describes ASD thus:[22][23]
[ASD] is characterised by persistent deficits in the ability to initiate and to sustain reciprocal social interaction and social communication, and by a range of restricted, repetitive, and inflexible patterns of behaviour, interests or activities that are clearly atypical or excessive for the individual's age and sociocultural context. The onset of the disorder occurs during the developmental period, typically in early childhood, but symptoms may not become fully manifest until later, when social demands exceed limited capacities. Deficits are sufficiently severe to cause impairment in personal, family, social, educational, occupational or other important areas of functioning and are usually a pervasive feature of the individual's functioning observable in all settings, although they may vary according to social, educational, or other context. Individuals along the spectrum exhibit a full range of intellectual functioning and language abilities.
— ICD-11, chapter 6, section A02
ICD-11 was produced by professionals from 55 countries out of the 90 countries involved and is the most widely used reference worldwide. Clinicians use the ICD as a reference for diagnosis and reporting but researchers, particularly in the US, continue to use the Diagnostic and Statistical Manual of Mental Disorders (DSM-5 (from 2013) or its predecessors) as some material is not included in the ICD (the ICD is also broader in scope, covering general as well as mental health). There remain differences, for example Rett disorder was included in ASD in the DSM-5 but in the ICD-11 it was excluded and placed in the chapter for Developmental Anomalies. Both the ICD and the DSM have been under revision and there has been collaborative work towards a convergence of the two since 1980 (when DSM-3 was published and ICD-9 was current), including more rigorous biological assessment - in place of historical experience - and a simplification of the system of classification.[23][24][25][26]
DSM
The American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders version 5 (DSM-5), released in May 2013, is the current version of the DSM, and is the first to define ASD as a single diagnosis.[27] ASD encompasses previous diagnoses which included Asperger disorder, childhood disintegrative disorder, PDD-NOS, and the range of diagnoses which included the word autism.[28]: 53 Rather than distinguishing between these diagnoses, the DSM-5 adopted a dimensional approach to diagnosing disorders that fall underneath the autistic spectrum umbrella because some proposed that individuals on the autism spectrum were better represented as a single diagnostic category. Within this category, the DSM-5 includes a framework that differentiates each individual by dimensions of severity, as well as by associated features (i.e., the presence of other disorders or factors which likely contribute to the symptoms, other neurodevelopmental or mental disorders, intellectual disability, or language impairment).[28]: 51, 53
Another change included collapsing social and communication deficits into one domain.[29] Thus, an individual with an ASD diagnosis would be described in terms of severity of social communication symptoms, severity of fixated or restricted behaviors or interests, and hyper- or hyposensitivity to sensory stimuli.
The restricting of onset age was also loosened from 3 years of age to "early developmental period", with a note that symptoms may manifest later when social demands exceed capabilities.[30]
Signs and symptoms
Clinicians consider assessment for ASD when a patient shows:
- regular difficulties in social interaction or communication
- restricted or repetitive behaviours (often called "stimming")
- resistance to changes or restricted interests
These features are typically assessed with the following, when appropriate:
- problems in obtaining or sustaining employment or education
- difficulties in initiating or sustaining social relationships
- connections with mental health or learning disability services
- a history of neurodevelopmental conditions (including learning disabilities and ADHD) or mental health conditions.[31][32]
There are many signs associated with ASD; the presentation varies widely:[33][34]
Common signs for autistic spectrum disorder - little or no infant babbling
- not pointing to show interest or not showing interest when something is indicated
- limited language skills e.g. having a smaller vocabulary than peers or difficulty expressing themselves in words
- having reduced interest in other children or carers, possibly with more interest in objects
- having trouble understanding other's feelings or talking about their own feelings
- showing interest in others, but not knowing how to play with them or relate to them
- avoiding playing pretending games
- unusual or limited usage of toys
- avoiding eye-contact
- wanting to be alone
- increased sensitivity to the smell, texture, sound, taste or appearance of things
- being upset by changes in routine, possibly with trouble adapting to the changes
- avoiding hugs, except when they want them
- difficulty expressing needs using everyday words or actions
- repeating words or phrases, or using them in place of typical language
- repeating actions over and over again
- making unusual movements, expressions, actions or positions
- apparently losing skills once held e.g. stopping using specific language
- self-harming
In addition, a small percentage of autistic people can exhibit notable ability, for example in mathematics, music or artistic reproduction, which in exceptional cases is referred to as savant syndrome.[35][36]
Developmental course
Most parents report that the onset of autism symptoms occur within the first year of life.[37][38] There are two possible developmental courses of ASD. One course of development is more gradual in nature, in which parents report concerns in development over the first two years of life and diagnosis is made around 3–4 years of age.[clarification needed] Some of the early signs of ASDs in this course include decreased looking at faces, failure to turn when name is called, failure to show interests by showing or pointing, and delayed imaginative play.[39]
A second course of development is characterized by normal or near-normal development before onset of regression or loss of skills. One pattern of regression occurs in the first 15 months to 3 years.[40][41] There are some who believe that regressive autism is simply early-onset autism that was recognized at a later date. Researchers have conducted studies to determine whether regressive autism is a distinct subset of ASD. Over the years, the results of these studies have contradicted one another. Some researchers believe there is still nothing to support a definitive biological difference between early-onset and regressive autism.[42] Another pattern, childhood disintegrative disorder (a DSM-IV diagnosis now included under ASD in DSM-5), is characterized by regression after normal development in the first 3 to 4, or even up to 9 years of life.[43]
Regressive autism
Regressive autism occurs when a child appears to develop typically but then starts to lose speech and social skills, typically between the ages of 15 and 30 months, and is subsequently diagnosed with autism.[42] Other terms used to describe regression in children with autism are autism with regression, autistic regression, setback-type autism, and acquired autistic syndrome.[44] There is no standard definition for regression,[44] and the prevalence of regression varies depending on the definition used.[45] Some children show a mixture of features, with some early delays and some later losses; and there is evidence of a continuous spectrum of behaviors, rather than, or in addition to, a black-and-white distinction, between autism with and without regression.[45] There are several intermediate types of development, which do not neatly fit into either the traditional early onset or the regressive categories, including mixtures of early deficits, failures to progress, subtle diminishments, and obvious losses. If regression is defined strictly to require loss of language, it is less common; if defined more broadly, to include cases where language is preserved but social interaction is diminished, it is more common.[45]
Regression may occur in a variety of domains, including communication, social, cognitive, and self-help skills; however, the most common regression is loss of language.[40][41] Some children lose social development instead of language; some lose both.[45] Skill loss may be quite rapid, or may be slow and preceded by a lengthy period of no skill progression; the loss may be accompanied by reduced social play or increased irritability.[44] The temporarily acquired skills typically amount to a few words of spoken language, and may include some rudimentary social perception.[45]
After the regression, the child follows the standard pattern of autistic neurological development. The term regressive autism refers to the appearance that neurological development has reversed; it is actually only the affected developmental skills, rather than the neurology as a whole, that regresses. It is more usual for autistic neurological development not to involve regression, with age-appropriate autistic symptoms being clear from birth.
The apparent onset of regressive autism is surprising and distressing to parents, who often initially suspect severe hearing loss.[citation needed] Attribution of regression to environmental stress factors may result in a delay in diagnosis.[46]
Differential outcomes
There continues to be a debate over the differential outcomes based on these two developmental courses. Some studies suggest that regression is associated with poorer outcomes and others report no differences between those with early gradual onset and those who experience a regression period.[47] While there is conflicting evidence surrounding language outcomes in ASD, some studies have shown that cognitive and language abilities at age 2+1⁄2 may help predict language proficiency and production after age 5.[48] Overall, the literature stresses the importance of early intervention in achieving positive longitudinal outcomes.[49]
Social and communication skills
Impairments in social skills present many challenges for autistic individuals. Deficits in social skills may lead to problems with friendships, romantic relationships, daily living, and vocational success.[50] One study that examined the outcomes of autistic adults found that, compared to the general population, autistic people were less likely to be married, but it is unclear whether this outcome was due to deficits in social skills or intellectual impairment, or some other reason.[51]
Prior to 2013, deficits in social function and communication were considered two separate symptoms of autism.[52] The current criteria for autism diagnosis require individuals to have deficits in three social skills: social-emotional reciprocity, nonverbal communication, and developing and sustaining relationships.[28] Communication deficits are due to problems with social-emotional skills like joint attention and social reciprocity.[medical citation needed]
A range of social-emotional reciprocity[clarification needed] symptoms may be present. Autistic individuals may lack mutual sharing of interests, for example many autistic children prefer not to play or interact with others. They may lack awareness or understanding of other people's thoughts or feelings - a child may get too close to peers (entering their personal space) without noticing that this makes them uncomfortable. They may also engage in atypical behaviors to gain attention, for example a child may push a peer to gain attention before starting a conversation.[53]
Autistic people experience deficits in their ability to develop, maintain, and understand relationships, as well as difficulties adjusting behavior to fit social contexts.[54] ASD presents with impairments in pragmatic communication skills, such as difficulty initiating a conversation or failure to consider the interests of the listener to sustain a conversation.[53][verification needed] The ability to be focused exclusively on one topic in communication is known as monotropism, and can be compared to "tunnel vision". It is common for autistic individuals to communicate strong interest in a specific topic, speaking in lesson-like monologues about their passion instead of enabling reciprocal communication with whomever they are speaking to.[55] What may look like self-involvement or indifference toward others stems from a struggle to recognize or remember that other people have their own personalities, perspectives, and interests.[56][57] Another difference in pragmatic communication skills is that autistic people may not recognise the need to control the volume of their voice in different social settings - for example, they may speak loudly in libraries or movie theaters.[58]
Autistic people display atypical nonverbal behaviours or have difficulties with nonverbal communication. They may make infrequent eye contact - an autistic child may not make eye contact when called by name, or they may avoid making eye contact with an observer. Aversion of gaze can also be seen in anxiety disorders, however poor eye contact in autistic children is not due to shyness or anxiety; rather, it is overall diminished in quantity. Autistic individuals may struggle with both production and understanding of facial expressions. They often do not know how to recognize emotions from others' facial expressions, or they may not respond with the appropriate facial expressions. They may have trouble recognizing subtle expressions of emotion and identifying what various emotions mean for the conversation.[59][55] They may also not pick up on body language or social cues such as eye contact and facial expressions if they provide more information than the person can process at that time. They struggle with understanding the context and subtext of conversational or printed situations, and have trouble forming resulting conclusions about the content. This also results in a lack of social awareness and atypical language expression.[56] How facial expressions differ between those on the autism spectrum and neurotypical individuals is not clear.[60] Further, at least half of autistic children have unusual prosody.[58]
Autistic people may also experience difficulties with verbal communication. Some autistic linguistic behaviors include repetitive or rigid language, and restricted interests in conversation. For example, a child might repeat words or insist on always talking about the same subject.[53] Echolalia may also be present in autistic individuals, for example by responding to a question by repeating the inquiry instead of answering.[55] Language impairment is also common in autistic children, but is not part of a diagnosis.[53] Many autistic children develop language skills at an uneven pace where they easily acquire some aspects of communication, while never fully developing others,[55] such as in some cases of hyperlexia. In some cases, individuals remain completely nonverbal throughout their lives. The CDC estimated that around 40% of autistic children don't speak at all, although the accompanying levels of literacy and nonverbal communication skills vary.[61]
Behavioural characteristics
ASDs include a wide variety of characteristics. Some of these include behavioural characteristics which widely range from slow development of social and learning skills to difficulties creating connections with other people. They may develop these difficulties of creating connections due to anxiety or depression, which autistic people are more likely to experience, and as a result isolate themselves.[62][medical citation needed]
Other behavioural characteristics include abnormal responses to sensations including sights, sounds, touch, taste and smell, and problems keeping a consistent speech rhythm. The latter problem influences an individual's social skills, leading to potential problems in how they are understood by communication partners. Behavioural characteristics displayed by autistic people typically influence development, language, and social competence. Behavioural characteristics of autistic people can be observed as perceptual disturbances, disturbances of development rate, relating, speech and language, and motility.[63]
The second core symptom of autism spectrum is a pattern of restricted and repetitive behaviors, activities, and interests. In order to be diagnosed with ASD, a person must have at least two of the following behaviors:[28][64]
- Repetitive behaviors – Repetitive behaviors such as rocking, hand flapping, finger flicking, head banging, or repeating phrases or sounds.[53] These behaviors may occur constantly or only when the child gets stressed, anxious or upset.
- Resistance to change – A strict adherence to routines such as eating certain foods in a specific order, or taking the same path to school every day.[53] The child may have a meltdown if there is any change or disruption to their routine.
- Restricted interests – An excessive interest in a particular activity, topic, or hobby, and devoting all their attention to it. For example, young children might completely focus on things that spin and ignore everything else. Older children might try to learn everything about a single topic, such as the weather or sports, and perseverate or talk about it constantly.[53]
- Sensory reactivity– An unusual reaction to certain sensory inputs such as having a negative reaction to specific sounds or textures, being fascinated by lights or movements or having an apparent indifference to pain or heat.
Self-injury
Self-injurious behaviors (SIB) are relatively common in autistic people, and can include head-banging, self-cutting, self-biting, and hair-pulling.[65] Some of these behaviors can result in serious injury or death.[65] Following are theories about the cause of self-injurious behavior in children with developmental delay, including autistic individuals:[66]
- Frequency and/or continuation of self-injurious behavior can be influenced by environmental factors (e.g. reward in return for halting self-injurious behavior). However this theory is not applicable to younger children with autism. There is some evidence that frequency of self-injurious behavior can be reduced by removing or modifying environmental factors that reinforce this behavior.[66]: 10–12
- Higher rates of self-injury are also noted in socially isolated individuals with autism. [citation needed]
- Self-injury could be a response to modulate pain perception when chronic pain or other health problems that cause pain are present.[66]: 12–13
- An abnormal basal ganglia connectivity may predispose to self-injurious behavior.[66]: 13
Causes
While specific causes of ASDs have yet to be found, many risk factors identified in the research literature may contribute to their development. These risk factors include genetics, prenatal and perinatal factors, neuroanatomical abnormalities, and environmental factors. It is possible to identify general risk factors, but much more difficult to pinpoint specific factors. Given the current state of knowledge, prediction can only be of a global nature and therefore requires the use of general markers.[67]
Genetics
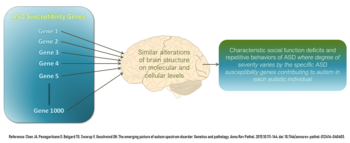
As of 2018[update], it appeared that between 74% and 93% of ASD risk is heritable.[64] After an older child is diagnosed with ASD, 7–20% of subsequent children are likely to be as well.[64] If parents have one autistic child, they have a 2% to 8% chance of having a second child who is also autistic. If the autistic child is an identical twin the other will be affected 36 to 95 percent of the time. If they are fraternal twins the other will only be affected up to 31 percent of the time.[medical citation needed]
As of 2018[update], understanding of genetic risk factors had shifted from a focus on a few alleles to an understanding that genetic involvement in ASD is probably diffuse, depending on a large number of variants, some of which are common and have a small effect, and some of which are rare and have a large effect. The most common gene disrupted with large effect rare variants appeared to be CHD8, but less than 0.5% of autistic people have such a mutation. The gene CHD8 encodes the protein chromodomain helicase DNA binding protein 8, which is a chromatin regulator enzyme that is essential during fetal development, CHD8 is an ATP dependent enzyme[69][70][71]. The protein contains an Snf2 helicase domain that is responsible for the hydrolysis of ATP to ADP[72]. CHD8 encodes for a DNA helicase that function as a transcription repressor by remodeling chromatin structure by altering the position of nucleosomes. CHD8 negatively regulates Wnt signaling. Wnt signaling is important in the vertebrate early development and morphogenesis. It is believed that CHD8 also recruits the linker histone H1 and causes the repression of β-catenin and p53 target genes[73]. The importance of CHD8 can be observed in studies where CHD8-knockout mice died after 5.5 embryonic days because of widespread p53 induced apoptosis. Some studies have determined the role of CHD8 in autism spectrum disorder (ASD). CHD8 expression significantly increases during human mid-fetal development[74]. The chromatin remodeling activity and its interaction with transcriptional regulators have shown to play an important role in ASD aetiology.[75] The developing mammalian brain has a conserved CHD8 target regions that are associated with ASD risk genes[76]. The knockdown of CHD8 in human neural stem cells results in dysregulation of ASD risk genes that are targeted by CHD8[77]. Recently CD8 has been asscociated to the regulation of long non-coding RNAs (lncRNAs)[78], and the regulation of X chromosome inactivation (XCI) initiation, via regulation of Xist long non-coding RNA, the master regulator of XCI, though competitive binding to Xist regulatory regions[79].
Some ASD is associated with clearly genetic conditions, like fragile X syndrome; however, only around 2% of autistic people have fragile X.[64] Hypotheses from evolutionary psychiatry suggest that these genes persist because they are linked to human inventiveness, intelligence or systemising.[80][81]
Current research suggests that genes that increase susceptibility to ASD are ones that control protein synthesis in neuronal cells in response to cell needs, activity and adhesion of neuronal cells, synapse formation and remodeling, and excitatory to inhibitory neurotransmitter balance. Therefore, despite up to 1000 different genes thought to contribute to increased risk of ASD, all of them eventually affect normal neural development and connectivity between different functional areas of the brain in a similar manner that is characteristic of an ASD brain. Some of these genes are known to modulate production of the GABA neurotransmitter which is the main inhibitory neurotransmitter in the nervous system. These GABA-related genes are underexpressed in an ASD brain. On the other hand, genes controlling expression of glial and immune cells in the brain e.g. astrocytes and microglia, respectively, are overexpressed which correlates with increased number of glial and immune cells found in postmortem ASD brains. Some genes under investigation in ASD pathophysiology are those that affect the mTOR signaling pathway which supports cell growth and survival.[82]
All these genetic variants contribute to the development of the autistic spectrum; however, it cannot be guaranteed that they are determinants for the development.[83]
Early life
Several prenatal and perinatal complications have been reported as possible risk factors for autism. These risk factors include maternal gestational diabetes, maternal and paternal age over 30, bleeding after first trimester, use of prescription medication (e.g. valproate) during pregnancy, and meconium in the amniotic fluid. While research is not conclusive on the relation of these factors to autism, each of these factors has been identified more frequently in children with autism, compared to their siblings who do not have autism, and other typically developing youth.[84] While it is unclear if any single factors during the prenatal phase affect the risk of autism,[85] complications during pregnancy may be a risk.[85]
Low vitamin D levels in early development has been hypothesized as a risk factor for autism.[86]
There are also studies being done to test if certain types of regressive autism have an autoimmune basis.[42]
Etiological hypotheses
Several hypotheses have been presented that try to explain how and why autism develops by integrating known causes (genetic and environmental effects) and findings (neurobiological and somatic). Some are more comprehensive, such as the Pathogenetic Triad,[87] which proposes and operationalises three core features (an autistic personality, cognitive compensation, neuropathological burden) that interact to cause autism, and the Intense World Theory,[88] which explains autism through a hyper-active neurobiology that leads to an increased perception, attention, memory, and emotionality. There are also simpler hypotheses that explain only individual parts of the neurobiology or phenotype of autism, such as mind-blindness (a decreased ability for Theory of Mind), the weak central coherence theory, or the extreme male brain and empathising-systemising theory.
Disproven vaccine hypothesis
In 1998 Andrew Wakefield led a fraudulent study that suggested that the MMR vaccine may cause autism.[89][90][91][92][93] This conjecture suggested that autism results from brain damage caused either by the MMR vaccine itself, or by thimerosal, a vaccine preservative.[94] No convincing scientific evidence supports these claims, and further evidence continues to refute them, including the observation that the rate of autism continues to climb despite elimination of thimerosal from routine childhood vaccines.[95] A 2014 meta-analysis examined ten major studies on autism and vaccines involving 1.25 million children worldwide; it concluded that neither the MMR vaccine, which has never contained thimerosal,[96] nor the vaccine components thimerosal or mercury, lead to the development of ASDs.[97]
Pathophysiology
In general, neuroanatomical studies support the concept that autism may involve a combination of brain enlargement in some areas and reduction in others.[98] These studies suggest that autism may be caused by abnormal neuronal growth and pruning during the early stages of prenatal and postnatal brain development, leaving some areas of the brain with too many neurons and other areas with too few neurons.[99] Some research has reported an overall brain enlargement in autism, while others suggest abnormalities in several areas of the brain, including the frontal lobe, the mirror neuron system, the limbic system, the temporal lobe, and the corpus callosum.[100][101]
In functional neuroimaging studies, when performing theory of mind and facial emotion response tasks, the median person on the autism spectrum exhibits less activation in the primary and secondary somatosensory cortices of the brain than the median member of a properly sampled control population. This finding coincides with reports demonstrating abnormal patterns of cortical thickness and grey matter volume in those regions of autistic persons' brains.[102]
Brain connectivity
Brains of autistic individuals have been observed to have abnormal connectivity and the degree of these abnormalities directly correlates with the severity of autism. Following are some observed abnormal connectivity patterns in autistic individuals:[103][82] '
- Decreased connectivity between different specialized regions of the brain (e.g. lower neuron density in corpus callosum) and relative overconnectivity within specialized regions of the brain by adulthood. Connectivity between different regions of the brain ('long-range' connectivity) is important for integration and global processing of information and comparing incoming sensory information with the existing model of the world within the brain. Connections within each specialized regions ('short-range' connections) are important for processing individual details and modifying the existing model of the world within the brain to more closely reflect incoming sensory information. In infancy, children at high risk for autism that were later diagnosed with autism were observed to have abnormally high long-range connectivity which then decreased through childhood to eventual long-range underconnectivity by adulthood.[103]
- Abnormal preferential processing of information by the left hemisphere of the brain vs. preferential processing of information by right hemisphere in neurotypical individuals. The left hemisphere is associated with processing information related to details whereas the right hemisphere is associated with processing information in a more global and integrated sense that is essential for pattern recognition. For example, visual information like face recognition is normally processed by the right hemisphere which tends to integrate all information from an incoming sensory signal, whereas an ASD brain preferentially processes visual information in the left hemisphere where information tends to be processed for local details of the face rather than the overall configuration of the face. This left lateralization negatively impacts both facial recognition and spatial skills.[103]
- Increased functional connectivity within the left hemisphere which directly correlates with severity of autism. This observation also supports preferential processing of details of individual components of sensory information over global processing of sensory information in an ASD brain.[103]
- Prominent abnormal connectivity in the frontal and occipital regions. In autistic individuals low connectivity in the frontal cortex was observed from infancy through adulthood. This is in contrast to long-range connectivity which is high in infancy and low in adulthood in ASD.[103] Abnormal neural organization is also observed in the Broca's area which is important for speech production.[82]
Neuropathology
Listed below are some characteristic findings in ASD brains on molecular and cellular levels regardless of the specific genetic variation or mutation contributing to autism in a particular individual:
- Limbic system with smaller neurons that are more densely packed together. Given that the limbic system is the main center of emotions and memory in the human brain, this observation may explain social impairment in ASD.[82]
- Fewer and smaller Purkinje neurons in the cerebellum. New research suggest a role of the cerebellum in emotional processing and language.[82]
- Increased number of astrocytes and microglia in the cerebral cortex. These cells provide metabolic and functional support to neurons and act as immune cells in the nervous system, respectively.[82]
- Increased brain size in early childhood causing macrocephaly in 15–20% of ASD individuals. The brain size however normalizes by mid-childhood. This variation in brain size in not uniform in the ASD brain with some parts like the frontal and temporal lobes being larger, some like the parietal and occipital lobes being normal sized, and some like cerebellar vermis, corpus callosum, and basal ganglia being smaller than neurotypical individuals.[82]
- Cell adhesion molecules that are essential to formation and maintenance of connections between neurons, neuroligins found on postsynaptic neurons that bind presynaptic cell adhesion molecules, and proteins that anchor cell adhesion molecules to neurons are all found to be mutated in ASD.[82]
Gut-immune-brain axis
Up to 70% of autistic individuals have GI related problems like reflux, diarrhea, constipation, inflammatory bowel disease, and food allergies.[citation needed] It has been observed that the makeup of gut bacteria in autistic people is different than that of neurotypical individuals which has raised the question of influence of gut bacteria on ASD development via inducing an inflammatory state.[104]
Listed below are some research findings on the influence of gut bacteria and abnormal immune responses on brain development:[104]
- Some studies on rodents have shown gut bacteria influencing emotional functions and neurotransmitter balance in the brain, both of which are impacted in ASD.[82]
- The immune system is thought to be the intermediary that modulates the influence of gut bacteria on the brain. Some ASD individuals have a dysfunctional immune system with higher numbers of some types of immune cells, biochemical messengers and modulators, and autoimmune antibodies. Increased inflammatory biomarkers correlate with increased severity of ASD symptoms and there is some evidence to support a state of chronic brain inflammation in ASD.[104]
- More pronounced inflammatory responses to bacteria were found in ASD individuals with an abnormal gut microbiota. Additionally, immunoglobulin A antibodies that are central to gut immunity were also found in elevated levels in ASD populations. Some of these antibodies may attack proteins that support myelination of the brain, a process that is important for robust transmission of neural signal in many nerves.[104]
- Activation of the maternal immune system during pregnancy (by gut bacteria, bacterial toxins, an infection, or non-infectious causes) and gut bacteria in the mother that induce increased levels of Th17, a proinflammatory immune cell, have been associated with an increased risk of autism. Some maternal IgG antibodies that cross the placenta to provide passive immunity to the fetus can also attack the fetal brain.[104]
- It is proposed that inflammation within the brain promoted by inflammatory responses to harmful gut microbiome impacts brain development.[104]
- Proinflammatory cytokines IFN-γ, IFN-α, TNF-α, IL-6 and IL-17 have been shown to promote autistic behaviors in animal models. Giving anti-IL-6 and anti-IL-17 along with IL-6 and IL-17, respectively, have been shown to negate this effect in the same animal models.[104]
- Some gut proteins and microbial products can cross the blood–brain barrier and activate mast cells in the brain. Mast cells release proinflammatory factors and histamine which further increase blood–brain barrier permeability and help set up a cycle of chronic inflammation.[104]
Mirror neuron system
The mirror neuron system consists of a network of brain areas that have been associated with empathy processes in humans.[105] In humans, the mirror neuron system has been identified in the inferior frontal gyrus and the inferior parietal lobule and is thought to be activated during imitation or observation of behaviors.[106] The connection between mirror neuron dysfunction and autism is tentative, and it remains to be seen how mirror neurons may be related to many of the important characteristics of autism.[107][108]
"Social brain" interconnectivity
A number of discrete brain regions and networks among regions that are involved in dealing with other people have been discussed together under the rubric of the "social brain". As of 2012[update], there is a consensus that autism spectrum is likely related to problems with interconnectivity among these regions and networks, rather than problems with any specific region or network.[109]
Temporal lobe
Functions of the temporal lobe are related to many of the deficits observed in individuals with ASDs, such as receptive language, social cognition, joint attention, action observation, and empathy. The temporal lobe also contains the superior temporal sulcus and the fusiform face area, which may mediate facial processing. It has been argued that dysfunction in the superior temporal sulcus underlies the social deficits that characterize autism. Compared to typically developing individuals, one study found that individuals with so-called 'high-functioning autism' had reduced activity in the fusiform face area when viewing pictures of faces.[110][verification needed]
Mitochondria
ASD could be linked to mitochondrial disease, a basic cellular abnormality with the potential to cause disturbances in a wide range of body systems.[111] A 2012 meta-analysis study, as well as other population studies show that approximately 5% of autistic children meet the criteria for classical mitochondrial dysfunction.[112] It is unclear why this mitochondrial disease occurs, considering that only 23% of children with both ASD and mitochondrial disease present with mitochondrial DNA abnormalities.[112]
Serotonin
Serotonin is a major neurotransmitter in the nervous system and contributes to formation of new neurons (neurogenesis), formation of new connections between neurons (synaptogenesis), remodeling of synapses, and survival and migration of neurons, processes that are necessary for a developing brain and some also necessary for learning in the adult brain. 45% of ASD individuals have been found to have increased blood serotonin levels.[82] It has been hypothesized that increased activity of serotonin in the developing brain may facilitate the onset of ASD, with an association found in six out of eight studies between the use of selective serotonin reuptake inhibitors (SSRIs) by the pregnant mother and the development of ASD in the child exposed to SSRI in the antenatal environment. The study could not definitively conclude SSRIs caused the increased risk for ASD due to the biases found in those studies, and the authors called for more definitive, better conducted studies.[113] Confounding by indication has since then been shown to be likely.[114] However, it is also hypothesized that SSRIs may help reduce symptoms of ASD and even positively affect brain development in some ASD patients.[82]
Diagnosis
This section needs to be updated. The reason given is: old sources, pre-DSM5. (March 2021) |

ASD can be detected as early as 18 months or even younger in some cases.[115] A reliable diagnosis can usually be made by the age of two years, however, because of delays in seeking and administering assessments, diagnoses often occur much later.[116] A study in 2005 found that children diagnosed with autistic disorder and PDD-NOS are typically diagnosed at age 3 while children with Aspergers are typically diagnosed at age 7.[117]
The diverse expressions of ASD behavioral and observational symptoms and absence of one specific genetic or molecular marker for the disorder pose diagnostic challenges to clinicians who use assessment methods based on symptoms alone. Individuals with an ASD may present at various times of development (e.g., toddler, child, or adolescent), and symptom expression may vary over the course of development.[118] Furthermore, clinicians who use those methods must differentiate among pervasive developmental disorders, and may also consider similar conditions, including intellectual disability not associated with a pervasive developmental disorder, specific language disorders, ADHD, anxiety, and psychotic disorders.[119] Ideally the diagnosis of ASD should be given by a team of professionals from different disciplines (e.g. child psychiatrists, child neurologists, psychologists) and only after the child has been observed in many different settings.[120]
Considering the unique challenges in diagnosing ASD using behavioral and observational assessment, specific practice parameters for its assessment were published by the American Academy of Neurology in the year 2000,[121] the American Academy of Child and Adolescent Psychiatry in 1999,[118] and a consensus panel with representation from various professional societies in 1999.[122] The practice parameters outlined by these societies include an initial screening of children by general practitioners (i.e., "Level 1 screening") and for children who fail the initial screening, a comprehensive diagnostic assessment by experienced clinicians (i.e. "Level 2 evaluation"). Furthermore, it has been suggested that assessments of children with suspected ASD be evaluated within a developmental framework, include multiple informants (e.g., parents and teachers) from diverse contexts (e.g., home and school), and employ a multidisciplinary team of professionals (e.g., clinical psychologists, neuropsychologists, and psychiatrists).[123]
As of 2019[update], psychologists would wait until a child showed initial evidence of ASD tendencies, then administer various psychological assessment tools to assess for ASD.[123] Among these measurements, the Autism Diagnostic Interview-Revised (ADI-R) and the Autism Diagnostic Observation Schedule (ADOS) are considered the "gold standards" for assessing autistic children.[124][125] The ADI-R is a semi-structured parent interview that probes for symptoms of autism by evaluating a child's current behavior and developmental history. The ADOS is a semistructured interactive evaluation of ASD symptoms that is used to measure social and communication abilities by eliciting several opportunities (or "presses") for spontaneous behaviors (e.g., eye contact) in standardized context. Various other questionnaires (e.g., The Childhood Autism Rating Scale, Autism Treatment Evaluation Checklist) and tests of cognitive functioning (e.g., The Peabody Picture Vocabulary Test) are typically included in an ASD assessment battery.
Screening
US and UK screening recommendations for autism in children younger than 3 years are:
- US Preventive Services Task Force does not recommend universal screen of young children for autism due to poor evidence of benefits of this screening when parents and clinicians have no concerns about ASD. The major concern is a false-positive diagnosis that would burden a family with very time-consuming and financially demanding treatment interventions when it is not truly required. The Task Force also did not find any robust studies showing effectiveness of behavioral therapies in reducing ASD symptom severity[126]
- American Academy of Pediatrics recommends ASD screening of all children between the ages if 18 and 24 months.[126] The AAP also recommends that children who screen positive for ASD be referred to treatment services without waiting for a comprehensive diagnostic workup[127]
- The American Academy of Family Physicians did not find sufficient evidence of benefit of universal early screening for ASD[126]
- The American Academy of Neurology and Child Neurology Society recommends general routine screening for delayed or abnormal development in children followed by screening for ASD only if indicated by the general developmental screening[126]
- The American Academy of Child and Adolescent Psychiatry recommend routinely screening autism symptoms in young children[126]
- The UK National Screening Committee does not recommend universal ASD screening in young children. Their main concerns includes higher chances of misdiagnosis at younger ages and lack of evidence of effectiveness of early interventions[126]
Misdiagnosis
There is a significant level of misdiagnosis of autism in neurodevelopmentally typical children; 18–37% of children diagnosed with ASD eventually lose their diagnosis. This high rate of lost diagnosis cannot be accounted for by successful ASD treatment alone. The most common reason parents reported as the cause of lost ASD diagnosis was new information about the child (73.5%), such as a replacement diagnosis. Other reasons included a diagnosis given so the child could receive ASD treatment (24.2%), ASD treatment success or maturation (21%), and parents disagreeing with the initial diagnosis (1.9%).[127][non-primary source needed]
Many of the children who were later found not to meet ASD diagnosis criteria then received diagnosis for another developmental disorder. Most common was ADHD, but other diagnoses included sensory disorders, anxiety, personality disorder, or learning disability.[127][non-primary source needed] Neurodevelopmental and psychiatric disorders that are commonly misdiagnosed as ASD include specific language impairment, social communication disorder, anxiety disorder, reactive attachment disorder, cognitive impairment, visual impairment, hearing loss and normal behavioral variation.[128] Some behavioral variations that resemble autistic traits are repetitive behaviors, sensitivity to change in daily routines, focused interests, and toe-walking. These are considered normal behavioral variations when they do not cause impaired function. Boys are more likely to exhibit repetitive behaviors especially when excited, tired, bored, or stressed. Some ways of distinguishing typical behavioral variations from autistic behaviors are the ability of the child to suppress these behaviors and the absence of these behaviors during sleep.[120]
Comorbidity
ASDs tend to be highly comorbid with other disorders. Comorbidity may increase with age and may worsen the course of youth with ASDs and make intervention/treatment more difficult. Distinguishing between ASDs and other diagnoses can be challenging because the traits of ASDs often overlap with symptoms of other disorders, and the characteristics of ASDs make traditional diagnostic procedures difficult.[129][130]
- The most common medical condition occurring in individuals with ASDs is seizure disorder or epilepsy, which occurs in 11–39% of autistic individuals.[131]
- Tuberous sclerosis, an autosomal dominant genetic condition in which non-malignant tumors grow in the brain and on other vital organs, is present in 1–4% of individuals with ASDs.[132]
- Intellectual disabilities are some of the most common comorbid disorders with ASDs. Recent estimates suggest that 40–69% of autistic individuals have some degree of an intellectual disability,[47] more likely to be severe for females. A number of genetic syndromes causing intellectual disability may also be comorbid with ASD, including fragile X, Down, Prader-Willi, Angelman, Williams syndrome[133] and SYNGAP1-related intellectual disability.[134][135]
- Learning disabilities are also highly comorbid in individuals with an ASD. Approximately 25–75% of individuals with an ASD also have some degree of a learning disability.[136]
- Various anxiety disorders tend to co-occur with ASDs, with overall comorbidity rates of 7–84%.[47] Rates of comorbid depression in individuals with an ASD range from 4–58%.[137] The relationship between ASD and schizophrenia remains a controversial subject under continued investigation, and recent meta-analyses have examined genetic, environmental, infectious, and immune risk factors that may be shared between the two conditions.[138][139][140] Oxidative stress, DNA damage and DNA repair have been postulated to play a role in the aetiopathology of both ASD and schizophrenia.[141]
- Deficits in ASD are often linked to behavior problems, such as difficulties following directions, being cooperative, and doing things on other people's terms.[142] Symptoms similar to those of attention deficit hyperactivity disorder (ADHD) can be part of an ASD diagnosis.[143]
- Sensory processing disorder is also comorbid with ASD, with comorbidity rates of 42–88%.[144]
- Starting in adolescence, some people with Asperger syndrome (26% in one sample)[145] fall under the criteria for the similar condition schizoid personality disorder, which is characterised by a lack of interest in social relationships, a tendency towards a solitary or sheltered lifestyle, secretiveness, emotional coldness, detachment and apathy.[145][146][147] Asperger syndrome was traditionally called "schizoid disorder of childhood".
Management
There is no known cure for autism, nor can any known treatments significantly reduce brain mutations caused by autism, although those with Asperger syndrome and those who have autism and require little-to-no support are more likely to experience a lessening of symptoms over time.[148][149][150] Several interventions can help children with autism.[151] The main goals of treatment are to lessen associated deficits and family distress, and to increase quality of life and functional independence. In general, higher IQs are correlated with greater responsiveness to treatment and improved treatment outcomes.[152][153] Although evidence-based interventions for autistic children vary in their methods, many adopt a psychoeducational approach to enhancing cognitive, communication, and social skills while minimizing problem behaviors. It has been argued that no single treatment is best and treatment is typically tailored to the child's needs.[154]
Non-pharmacological interventions
Intensive, sustained special education or remedial education programs and behavior therapy early in life can help children acquire self-care, social, and job skills. Available approaches include applied behavior analysis, developmental models, structured teaching, speech and language therapy, social skills therapy, and occupational therapy.[154] Among these approaches, interventions either treat autistic features comprehensively, or focus treatment on a specific area of deficit.[153] Generally, when educating those with autism, specific tactics may be used to effectively relay information to these individuals. Using as much social interaction as possible is key in targeting the inhibition autistic individuals experience concerning person-to-person contact. Additionally, research has shown that employing semantic groupings, which involves assigning words to typical conceptual categories, can be beneficial in fostering learning.[155]
There has been increasing attention to the development of evidence-based interventions for autistic young children. Two theoretical frameworks outlined for early childhood intervention include applied behavioral analysis (ABA) and the developmental social-pragmatic model (DSP).[153] Although ABA therapy has a strong evidence base, particularly in regard to early intensive home-based therapy, ABA's effectiveness may be limited by diagnostic severity and IQ of the person affected by ASD.[156] The Journal of Clinical Child and Adolescent Psychology has deemed two early childhood interventions as "well-established": individual comprehensive ABA, and focused teacher-implemented ABA combined with DSP.[153]
Another evidence-based intervention that has demonstrated efficacy is a parent training model, which teaches parents how to implement various ABA and DSP techniques themselves.[153] Various DSP programs have been developed to explicitly deliver intervention systems through at-home parent implementation.
A multitude of unresearched alternative therapies have also been implemented. Many have resulted in harm to autistic people and should not be employed unless proven to be safe.[154] However, a recent systematic review on adults with autism has provided emerging evidence for decreasing stress, anxiety, ruminating thoughts, anger, and aggression through mindfulness-based interventions for improving mental health.[157]
In October 2015, the American Academy of Pediatrics (AAP) proposed new evidence-based recommendations for early interventions in ASD for children under 3.[158] These recommendations emphasize early involvement with both developmental and behavioral methods, support by and for parents and caregivers, and a focus on both the core and associated symptoms of ASD.[158] However, a Cochrane review found no evidence that early intensive behavioral intervention (EIBI) is effective in reducing behavioral problems associated with autism in most autistic children but did help improve IQ and language skills. The Cochrane review did acknowledge that this may be due to the low quality of studies currently available on EIBI and therefore providers should recommend EIBI based on their clinical judgement and the family's preferences. No adverse effects of EIBI treatment were found.[159] Studies on pet therapy have shown positive effects.[160]
Generally speaking, treatment of ASD focuses on behavioral and educational interventions to target its two core symptoms: social communication deficits and restricted, repetitive behaviors.[161] If symptoms continue after behavioral strategies have been implemented, some medications can be recommended to target specific symptoms or co-existing problems such as restricted and repetitive behaviors (RRBs), anxiety, depression, hyperactivity/inattention and sleep disturbance.[161] Melatonin for example can be used for sleep problems.[162]
While there are a number of parent-mediated behavioral therapies to target social communication deficits in children with autism, there is uncertainty regarding the efficacy of interventions to treat RRBs.[163]
Pharmacological interventions
There is some emerging data that show positive effects of risperidone on restricted and repetitive behaviors (i.e., stimming; e.g., flapping, twisting, complex whole-body movements), but due to the small sample size of these studies and the concerns about its side effects, antipsychotics are not recommended as primary treatment of RRBs.[164]
Epidemiology
According to the WHO, about 1 in 100 children have autism.[165]
While rates of ASD are consistent across cultures, they vary greatly by gender, with boys diagnosed far more frequently than girls. The average male-to-female diagnosis ratio for ASD is 4.2:1,[166] with 1 in 70 boys, but only 1 in 315 girls.[167] Girls, however, are more likely to have associated cognitive impairment, suggesting that less severe forms of ASD are likely being missed in girls and women.[168]: 6A02 Among those with an ASD and intellectual disability, the sex ratio may be closer to 2:1.[169] Prevalence differences may be a result of gender differences in expression of clinical symptoms, with women and girls with autism showing less atypical behaviors and, therefore, less likely to receive an ASD diagnosis.[170]
Autism prevalence has been estimated at 1–2 per 1,000, Asperger syndrome at roughly 0.6 per 1,000, childhood disintegrative disorder at 0.02 per 1,000, and PDD-NOS at 3.7 per 1,000.[171] These rates are consistent across cultures and ethnic groups, as autism is considered a universal disorder.[47]
Using DSM-5 criteria 92% of the children diagnosed per DSM-IV with one of the disorders which is now considered part of ASD will still meet the diagnostic criteria of ASD. However, if both ASD and Social Communication Disorder categories of DSM-5 are combined, the prevalence of autism is mostly unchanged from the prevalence per the DSM-IV criteria. The best estimate for prevalence of ASD is 0.7% or 1 child in 143 children.[172] Relatively mild forms of autism, such as Aspergers as well as other developmental disorders were included in the recent DSM-5 diagnostic criteria.[173] ASD rates were constant between 2014 and 2016 but twice the rate compared to the time period between 2011 and 2014 (1.25 vs 2.47%). A Canadian meta-analysis from 2019 confirmed these effects as the profiles of people diagnosed with autism became less and less different from the profiles of the general population.[174] In the US, the rates for diagnosed ASD have been steadily increasing since 2000 when records began being kept.[175] While it remains unclear whether this trend represents a true rise in incidence, it likely reflects changes in ASD diagnostic criteria, improved detection, and increased public awareness of autism.[176]
United States
In the United States it is estimated to affect more than 2% of children (about 1.5 million) as of 2016[update].[177] According to the latest CDC prevalence reports, 1 in 44 children (2.3%) in the United States had a diagnosis of ASD in 2018.[178] Prevalence is estimated at 6 per 1,000 for ASDs as a whole,[171] although prevalence rates vary for each of the developmental disorders in the spectrum.
History
The word autism comes from the Greek word autos, whose meaning was first established by Eugen Bleuler.[179] Autism as it is known today can be drawn back to the late 1930s. Two separate psychiatrists used the word autism to describe the patients they were studying in their own clinical research. Victor, the wild boy of Aveyron, was found deep in the woods of Central France. He was non-verbal during his teenage years, and his case was widely popular among society for its time. Such cases brought awareness to autism, and more research was conducted on the natural dimensions of human behavior. The discussion of autism prior to the twentieth century is one that brings about much controversy. Without researchers being able to meet a consensus on the varying forms around the condition, there was a lack of research being conducted on the disorder. Discussing the syndrome and its complexity frustrated researchers. Controversies have surrounded various claims regarding the etiology of ASDs. In the 1950s, the refrigerator mother theory emerged as an explanation for autism. The hypothesis was based on the idea that autistic behaviors stem from the emotional frigidity, lack of warmth, and cold, distant, rejecting demeanor of a child's mother.[180] Parents of children with an ASD experienced blame, guilt, and self-doubt, especially as the theory was embraced by the medical establishment and went largely unchallenged into the mid-1960s.[citation needed] The "refrigerator mother" theory has since continued to be refuted in scientific literature, including a 2015 systematic review which showed no association between caregiver interaction and language outcomes in ASD.[181]
Leo Kanner, a child psychiatrist, was the first person to describe ASD as a neurodevelopmental disorder in 1943 by calling it infantile autism and therefore rejected the refrigerator mother theory.[182]
Another controversial claim suggests that watching extensive amounts of television may cause autism. This hypothesis was largely based on research suggesting that the increasing rates of autism in the 1970s and 1980s were linked to the growth of cable television at this time.[95]
Society and culture
Caregivers
Families who care for an autistic child face added stress from a number of different causes.[183] Parents may struggle to understand the diagnosis and to find appropriate care options. Parents often take a negative view of the diagnosis, and may struggle emotionally.[184] More than half of parents over the age of 50 are still living with their child as about 85% of autistic people have difficulties living independently.[185]
Neurodiversity movement

The neurodiversity movement is a social movement within the context of disability rights that emphasizes the concept of neurodiversity, viewing the autism spectrum as a result of natural variations in the human brain rather than a disorder to be cured.[186] The autism rights movement advocates for including greater acceptance of autistic behaviors; therapies that focus on coping skills rather than imitating the behaviors of those without autism;[187] and the recognition of the autistic community as a minority group.[187][188] Autism rights or neurodiversity advocates believe that the autism spectrum is genetic and should be accepted as a natural expression of the human genome. This perspective is distinct from two other likewise distinct views: the medical perspective, that autism is caused by a genetic defect and should be addressed by targeting the autism gene(s), and fringe theories that autism is caused by environmental factors such as vaccines.[186] Although these movements are not without criticism, for example, a common argument made against neurodiversity activists is that the majority of them are "high-functioning", or have Asperger syndrome, or who self-diagnose, and do not represent the views of "low-functioning" autistic people.[188][189][190] The concept is contentious within various autism advocacy and research groups and has led to infighting.[191][192][193]
Academic performance
The examples and perspective in this section deal primarily with United States and do not represent a worldwide view of the subject. (April 2022) |
The number of students identified and served as eligible for autism services in the United States has increased from 5,413 children in 1991–1992 to 370,011 children in the 2010–2011 academic school year.[194] The United States Department of Health and Human Services reported approximately 1 in 68 children are diagnosed with ASD at age 8 although onset is typically between ages 2 and 4.[194]
The increasing number of students diagnosed with ASD in the schools presents significant challenges to teachers, school psychologists, and other school professionals.[194] These challenges include developing a consistent practice that best support the social and cognitive development of the increasing number of autistic students.[194] Although there is considerable research addressing assessment, identification, and support services for autistic children, there is a need for further research focused on these topics within the school context.[194] Further research on appropriate support services for students with ASD will provide school psychologists and other education professionals with specific directions for advocacy and service delivery that aim to enhance school outcomes for students with ASD.[194]
Attempts to identify and use best intervention practices for students with autism also pose a challenge due to overdependence on popular or well-known interventions and curricula.[194] Some evidence suggests that although these interventions work for some students, there remains a lack of specificity for which type of student, under what environmental conditions (one-on-one, specialized instruction or general education) and for which targeted deficits they work best.[194] More research is needed to identify what assessment methods are most effective for identifying the level of educational needs for students with ASD.
A difficulty for academic performance in students with ASD is the tendency to generalize learning.[57] Learning is different for each student, which is the same for students with ASD. To assist in learning, accommodations are commonly put into place for students with differing abilities. The existing schema of these students works in different ways and can be adjusted to best support the educational development for each student.[195]
The cost of educating a student with ASD in the US is about $8,600 a year more than the cost of educating an average student, which is about $12,000.[196]
Employment
The examples and perspective in this section deal primarily with United States and do not represent a worldwide view of the subject. (April 2022) |
About half of people in their 20s with autism are unemployed, and one third of those with graduate degrees may be unemployed.[197] While employers state hiring concerns about productivity and supervision, experienced employers of autistics give positive reports of above average memory and detail orientation as well as a high regard for rules and procedure in autistic employees.[197] A majority of the economic burden of autism is caused by lost productivity in the job market.[198] Some studies also find decreased earning among parents who care for autistic children.[199][200] Adding content related to autism in existing diversity training can clarify misconceptions, support employees, and help provide new opportunities for autistic people.[201] As of 2021, the potential for new autism employment initiatives by major employers continue to grow. The most high-profile autism initiative in the United States, "Autism at Work" grew to 20 of the largest companies in the United States.[202]
See also
References
- ^ a b c d Bonati M, Cartabia M, Clavenna A (January 2022). "Still too much delay in recognition of autism spectrum disorder". Epidemiology and Psychiatric Sciences. 31 (e1). Cambridge University Press: e1. doi:10.1017/S2045796021000822. LCCN 2011243374. OCLC 727338545. PMC 8786613. PMID 35012703. S2CID 245851335.
- ^ a b Wing L, Gould J, Gillberg C (1 March 2011). "Autism spectrum disorders in the DSM-V: better or worse than the DSM-IV?". Research in Developmental Disabilities. 32 (2): 768–773. doi:10.1016/j.ridd.2010.11.003. PMID 21208775.
- ^ Rosen NE, Lord C, Volkmar FR (December 2021). "The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond". Journal of Autism and Developmental Disorders. 51 (12): 4253–4270. doi:10.1007/s10803-021-04904-1. PMC 8531066. PMID 33624215.
- ^ "WHO releases new International Classification of Diseases (ICD 11)". www.who.int. Retrieved 29 October 2021.
- ^ "DSM-5 Fact Sheets". www.psychiatry.org. Retrieved 6 March 2022.
- ^ Reed GM, First MB, Kogan CS, Hyman SE, Gureje O, Gaebel W, et al. (February 2019). "Innovations and changes in the ICD-11 classification of mental, behavioural and neurodevelopmental disorders". World Psychiatry. 18 (1): 3–19. doi:10.1002/wps.20611. PMC 6313247. PMID 30600616.
- ^ Ripamonti L (April 2016). "Disability, Diversity, and Autism: Philosophical Perspectives on Health". The New Bioethics. 22 (1): 56–70. doi:10.1080/20502877.2016.1151256. PMID 28219280. S2CID 23248611.
- ^ Pellicano E, den Houting J (April 2022). "Annual Research Review: Shifting from 'normal science' to neurodiversity in autism science". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 63 (4): 381–396. doi:10.1111/jcpp.13534. PMID 34730840. S2CID 241118562.
- ^ Fletcher-Watson S (2019). Autism : a new introduction to psychological theory and current debates. Francesca Happé (New & Updated ed.). Abingdon, Oxon: Routledge. p. 30. ISBN 978-1-315-10169-9. OCLC 1073035060.
- ^ Lai MC, Lombardo MV, Chakrabarti B, Baron-Cohen S (April 2013). "Subgrouping the autism "spectrum": reflections on DSM-5". PLoS Biology. 11 (4): e1001544. doi:10.1371/journal.pbio.1001544. PMC 3635864. PMID 23630456.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "What is Autistic Spectrum Disorder/Condition (ASD/C)?". psychiatry-uk.com. Retrieved 25 October 2021.
- ^ Walker N (2021). Neuroqueer heresies : notes on the neurodiversity paradigm, autistic empowerment, and postnormal possibilities. Fort Worth, TX. ISBN 978-1-945955-26-6. OCLC 1287945422.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ Bailin A. "Clearing Up Some Misconceptions about Neurodiversity". Scientific American Blog Network. Retrieved 17 March 2022.
- ^ Robison JE (2020). Kapp SK (ed.). My Time with Autism Speaks. Singapore: Springer. pp. 221–232. doi:10.1007/978-981-13-8437-0_16. ISBN 978-981-13-8437-0. S2CID 210496353. Retrieved 23 April 2022.
{{cite book}}:|work=ignored (help) - ^ "A medical condition or just a difference? The question roils autism community". Washington Post. ISSN 0190-8286. Retrieved 23 April 2022.
- ^ Mandy W, Lai MC (March 2016). "Annual Research Review: The role of the environment in the developmental psychopathology of autism spectrum condition". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 57 (3): 271–292. doi:10.1111/jcpp.12501. PMID 26782158.
- ^ Rosen NE, Lord C, Volkmar FR (December 2021). "The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond". Journal of Autism and Developmental Disorders. 51 (12): 4253–4270. doi:10.1007/s10803-021-04904-1. PMC 8531066. PMID 33624215.
- ^ Losh M, Adolphs R, Piven J. The Broad Autism Phenotype. Oxford University Press. doi:10.1093/med/9780195371826.003.0031. ISBN 978-0-19-996521-2.
- ^ Chapman R, Veit W (November 2021). "Correction to: The essence of autism: fact or artefact?". Molecular Psychiatry. 26 (11): 7069. doi:10.1038/s41380-021-01057-6. PMID 34697454. S2CID 239771302.
- ^ Wazana A, Bresnahan M, Kline J (June 2007). "The autism epidemic: fact or artifact?". Journal of the American Academy of Child and Adolescent Psychiatry. 46 (6): 721–730. doi:10.1097/chi.0b013e31804a7f3b. PMID 17513984.
- ^ "WHO releases new International Classification of Diseases (ICD 11)". World Health Organisation (Press Release). Retrieved 29 October 2021.
- ^ "Autism spectrum disorder". icd.who.int/en. Retrieved 22 October 2021.
- ^ a b Pickett D, Anderson RN (18 July 2018). Status on ICD-11: The WHO Launch (PDF) (Report). CDC/NCHS.
- ^ "ICD VS. DSM". APA Monitor. Vol. 40, no. 9. American Psychological Association. 2009. p. 63.
- ^ Mezzich JE (2002). "International surveys on the use of ICD-10 and related diagnostic systems". Psychopathology. 35 (2–3): 72–75. doi:10.1159/000065122. PMID 12145487. S2CID 35857872.
- ^ Goldberg D (January 2010). "The classification of mental disorder: a simpler system for DSM–V and ICD–11". Advances in Psychiatric Treatment. 16 (1): 14–19. doi:10.1192/apt.bp.109.007120.
- ^ "Home | APA DSM-5". Dsm5.org. Archived from the original on 19 November 2008. Retrieved 21 February 2012.
- ^ a b c d American Psychiatric Association (2013). "Autism Spectrum Disorder. 299.00 (F84.0)". Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Arlington, VA: American Psychiatric Publishing. pp. 50–59. doi:10.1176/appi.books.9780890425596. hdl:2027.42/138395. ISBN 978-0-89042-559-6.
- ^ Kulage KM, Smaldone AM, Cohn EG (August 2014). "How will DSM-5 affect autism diagnosis? A systematic literature review and meta-analysis". Journal of Autism and Developmental Disorders. 44 (8): 1918–32. doi:10.1007/s10803-014-2065-2. PMID 24531932. S2CID 18865395.
- ^ "DSM-5 Diagnostic Criteria". U.S. Department of Health & Human Services Interagency Autism Coordinating Committee. Retrieved 17 May 2017.
- ^ Autism spectrum disorder in adults: diagnosis and management, UK: NICE, 14 June 2021, CG142, retrieved 24 October 2021
- ^ "About autism spectrum disorder (ASD)". Government of Canada. 18 January 2016. Retrieved 4 November 2021.
- ^ "What are the signs and symptoms of ASD?". Government of Canada. 18 January 2016. Retrieved 4 November 2021.
- ^ National Center on Birth Defects and Developmental Disabilities (25 March 2020). "What is Autism Spectrum Disorder?". Centers for Disease Control and Prevention. US. Retrieved 24 October 2021.
- ^ Zhang Y, Han VZ (April 2018). "[Neurobiological mechanisms of autistic savant and acquired savant]". Sheng Li Xue Bao. 70 (2): 201–210. PMID 29691585.
- ^ Hughes JE, Ward J, Gruffydd E, Baron-Cohen S, Smith P, Allison C, Simner J (October 2018). "Savant syndrome has a distinct psychological profile in autism". Molecular Autism. 9: 53. doi:10.1186/s13229-018-0237-1. PMC 6186137. PMID 30344992.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, et al. (May 2009). "Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants". Pediatrics. 123 (5): 1383–91. doi:10.1542/peds.2008-1606. PMC 2833286. PMID 19403506.
- ^ Lord C (November 1995). "Follow-up of two-year-olds referred for possible autism". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 36 (8): 1365–82. doi:10.1111/j.1469-7610.1995.tb01669.x. PMID 8988272.
- ^ Zwaigenbaum L (October 2001). "Autistic spectrum disorders in preschool children". Canadian Family Physician. 47 (10): 2037–42. PMC 2018435. PMID 11723598.
- ^ a b Martínez-Pedraza F, Carter AS (July 2009). "Autism spectrum disorders in young children". Child and Adolescent Psychiatric Clinics of North America. 18 (3): 645–63. doi:10.1016/j.chc.2009.02.002. PMC 3166636. PMID 19486843.
- ^ a b Werner E, Dawson G, Munson J, Osterling J (June 2005). "Variation in early developmental course in autism and its relation with behavioral outcome at 3-4 years of age". Journal of Autism and Developmental Disorders. 35 (3): 337–50. doi:10.1007/s10803-005-3301-6. PMID 16119475. S2CID 22485657.
- ^ a b c Stefanatos GA (December 2008). "Regression in autistic spectrum disorders". Neuropsychology Review. 18 (4): 305–319. doi:10.1007/s11065-008-9073-y. PMID 18956241. S2CID 34658024.
- ^ "Autism spectrum disorder – childhood disintegrative disorder: MedlinePlus Medical Encyclopedia". medlineplus.gov. Retrieved 8 May 2020.
- ^ a b c Halsey NA, Hyman SL (May 2001). "Measles-mumps-rubella vaccine and autistic spectrum disorder: report from the New Challenges in Childhood Immunizations Conference convened in Oak Brook, Illinois, June 12-13, 2000". Pediatrics. 107 (5): E84. doi:10.1542/peds.107.5.e84. PMID 11331734.
- ^ a b c d e Ozonoff S, Heung K, Byrd R, Hansen R, Hertz-Picciotto I (December 2008). "The onset of autism: patterns of symptom emergence in the first years of life". Autism Research. 1 (6): 320–328. doi:10.1002/aur.53. PMC 2857525. PMID 19360687.
- ^ Johnson CP, Myers SM (2007). "Identification and evaluation of children with autism spectrum disorders". Pediatrics. 120 (5): 1183–215. doi:10.1542/peds.2007-2361. PMID 17967920.
- ^ a b c d Mash EJ, Barkley RA (2003). Child Psychopathology. New York: The Guilford Press. pp. 409–454. ISBN 9781572306097.
- ^ Ellis Weismer S, Kover ST (December 2015). "Preschool language variation, growth, and predictors in children on the autism spectrum". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 56 (12): 1327–37. doi:10.1111/jcpp.12406. PMC 4565784. PMID 25753577.
- ^ Dawson G, Osterling J (1997). "Early Intervention in Autism". In Guralnick MJ (ed.). The effectiveness of early intervention. Baltimore: Brookes. pp. 307–326. ISBN 1-55766-255-X. OCLC 34411043. ERIC ED414694.
- ^ Barnhill GP (2007). "Outcomes in adults with Asperger syndrome". Focus on Autism and Other Developmental Disabilities. 22 (2): 116–126. doi:10.1177/10883576070220020301. S2CID 1355689.
- ^ Howlin P, Moss P (May 2012). "Adults with autism spectrum disorders". Canadian Journal of Psychiatry. 57 (5): 275–83. doi:10.1177/070674371205700502. PMID 22546059. S2CID 44544407.
- ^ Frye RE (August 2018). "Social Skills Deficits in Autism Spectrum Disorder: Potential Biological Origins and Progress in Developing Therapeutic Agents". CNS Drugs. 32 (8): 713–734. doi:10.1007/s40263-018-0556-y. PMC 6105175. PMID 30105528.
- ^ a b c d e f g Augustyn M. "Autism spectrum disorder: Clinical features". UpToDate. Retrieved 22 March 2020.
- ^ National Center on Birth Defects and Developmental Disabilities (29 June 2020). "Diagnostic Criteria: Autism Spectrum Disorder (ASD)". Centers for Disease Control and Prevention. US. Retrieved 21 February 2021.
- ^ a b c d "Autism Spectrum Disorder: Communication Problems in Children". NIDCD. 18 August 2015. Retrieved 17 December 2017.
- ^ a b Vicker B. "Social communication and language characteristics associated with high-functioning, verbal children and adults with autism spectrum disorder". Indiana Resource Center for Autism. Retrieved 17 December 2017.
- ^ a b Lawson W (2001). Understanding and Working With the Spectrum of Autism An Insider's View. Philadelphia, PA: Jessica Kingsley Publishers London and Philadelphia. pp. 33. ISBN 978-1853029714.
- ^ a b Fusaroli R, Lambrechts A, Bang D, Bowler DM, Gaigg SB (March 2017). "Is voice a marker for Autism spectrum disorder? A systematic review and meta-analysis" (PDF). Autism Research. 10 (3): 384–407. doi:10.1002/aur.1678. PMID 27501063. S2CID 13772771.
- ^ "Autism: Overview". American Speech-Language-Hearing Association. Retrieved 17 December 2017.
- ^ Keating CT, Cook JL (July 2020). "Facial expression production and recognition in autism spectrum disorders: a shifting landscape". Child Adolesc Psychiatr Clin N Am (Review). 29 (3): 557–571. doi:10.1016/j.chc.2020.02.006. PMID 32471602.
- ^ National Center on Birth Defects and Developmental Disabilities (26 February 2015). "Signs & Symptoms: Autism Spectrum Disorder". Centers for Disease Control and Prevention. US. Archived from the original on 10 March 2015. (Also available in Spanish.)
- ^ "Engaging people on the autism spectrum". Autism Spectrum Australia. 7 October 2013.[dead link]
- ^ Hinerman PS (1983). Teaching Autistic Children to Communicate. Rockville, Maryland: Aspens System Corporation. p. 180. ISBN 978-0-89443-884-4.
- ^ a b c d Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J (August 2018). "Autism spectrum disorder". Lancet. 392 (10146): 508–520. doi:10.1016/S0140-6736(18)31129-2. PMC 7398158. PMID 30078460. S2CID 51922565.
- ^ a b Minshawi NF, Hurwitz S, Fodstad JC, Biebl S, Morriss DH, McDougle CJ (April 2014). "The association between self-injurious behaviors and autism spectrum disorders". Psychology Research and Behavior Management. 7: 125–36. doi:10.2147/PRBM.S44635. PMC 3990505. PMID 24748827.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b c d Oliver C, Richards C (October 2015). "Practitioner Review: Self-injurious behaviour in children with developmental delay" (PDF). J Child Psychol Psychiatry (Review). 56 (10): 1042–54. doi:10.1111/jcpp.12425. PMID 25916173.
- ^ Tager-Flusberg H (2010). "The origins of social impairments in autism spectrum disorder: studies of infants at risk". Neural Networks. 23 (8–9): 1072–6. doi:10.1016/j.neunet.2010.07.008. PMC 2956843. PMID 20800990.
- ^ Brown EA, Lautz JD, Davis TR, Gniffke EP, VanSchoiack AA, Neier SC, et al. (2018). "Clustering the autisms using glutamate synapse protein interaction networks from cortical and hippocampal tissue of seven mouse models". Molecular Autism. 9 (48): 48. doi:10.1186/s13229-018-0229-1. PMC 6139139. PMID 30237867.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Nishiyama, Masaaki; Oshikawa, Kiyotaka; Tsukada, Yu-ichi; Nakagawa, Tadashi; Iemura, Shun-ichiro; Natsume, Tohru; Fan, Yuhong; Kikuchi, Akira; Skoultchi, Arthur I.; Nakayama, Keiichi I. (18 January 2009). "CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis". Nature Cell Biology. 11 (2): 172–182. doi:10.1038/ncb1831. ISSN 1465-7392.
- ^ Ronan, Jehnna L.; Wu, Wei; Crabtree, Gerald R. (9 April 2013). "From neural development to cognition: unexpected roles for chromatin". Nature Reviews Genetics. 14 (5): 347–359. doi:10.1038/nrg3413. ISSN 1471-0056.
- ^ Thompson, Brandi A.; Tremblay, Véronique; Lin, Grace; Bochar, Daniel A. (15 June 2008). "CHD8 Is an ATP-Dependent Chromatin Remodeling Factor That Regulates β-Catenin Target Genes". Molecular and Cellular Biology. 28 (12): 3894–3904. doi:10.1128/mcb.00322-08. ISSN 0270-7306.
- ^ Thompson, Brandi A.; Tremblay, Véronique; Lin, Grace; Bochar, Daniel A. (15 June 2008). "CHD8 Is an ATP-Dependent Chromatin Remodeling Factor That Regulates β-Catenin Target Genes". Molecular and Cellular Biology. 28 (12): 3894–3904. doi:10.1128/mcb.00322-08. ISSN 0270-7306.
- ^ Nishiyama, Masaaki; Oshikawa, Kiyotaka; Tsukada, Yu-ichi; Nakagawa, Tadashi; Iemura, Shun-ichiro; Natsume, Tohru; Fan, Yuhong; Kikuchi, Akira; Skoultchi, Arthur I.; Nakayama, Keiichi I. (18 January 2009). "CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis". Nature Cell Biology. 11 (2): 172–182. doi:10.1038/ncb1831. ISSN 1465-7392.
- ^ Nishiyama, Masaaki; Oshikawa, Kiyotaka; Tsukada, Yu-ichi; Nakagawa, Tadashi; Iemura, Shun-ichiro; Natsume, Tohru; Fan, Yuhong; Kikuchi, Akira; Skoultchi, Arthur I.; Nakayama, Keiichi I. (18 January 2009). "CHD8 suppresses p53-mediated apoptosis through histone H1 recruitment during early embryogenesis". Nature Cell Biology. 11 (2): 172–182. doi:10.1038/ncb1831. ISSN 1465-7392.
- ^ Ronan, Jehnna L.; Wu, Wei; Crabtree, Gerald R. (9 April 2013). "From neural development to cognition: unexpected roles for chromatin". Nature Reviews Genetics. 14 (5): 347–359. doi:10.1038/nrg3413. ISSN 1471-0056.
- ^ Sugathan, Aarathi; Biagioli, Marta; Golzio, Christelle; Erdin, Serkan; Blumenthal, Ian; Manavalan, Poornima; Ragavendran, Ashok; Brand, Harrison; Lucente, Diane; Miles, Judith; Sheridan, Steven D. (7 October 2014). "CHD8 regulates neurodevelopmental pathways associated with autism spectrum disorder in neural progenitors". Proceedings of the National Academy of Sciences. 111 (42). doi:10.1073/pnas.1405266111. ISSN 0027-8424.
{{cite journal}}: line feed character in|title=at position 12 (help) - ^ Cotney, Justin; Muhle, Rebecca A.; Sanders, Stephan J.; Liu, Li; Willsey, A. Jeremy; Niu, Wei; Liu, Wenzhong; Klei, Lambertus; Lei, Jing; Yin, Jun; Reilly, Steven K. (10 March 2015). "The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment". Nature Communications. 6 (1). doi:10.1038/ncomms7404. ISSN 2041-1723.
- ^ Wilkinson, B; Grepo, N; Thompson, B L; Kim, J; Wang, K; Evgrafov, O V; Lu, W; Knowles, J A; Campbell, D B (2015-05). "The autism-associated gene chromodomain helicase DNA-binding protein 8 (CHD8) regulates noncoding RNAs and autism-related genes". Translational Psychiatry. 5 (5): e568–e568. doi:10.1038/tp.2015.62. ISSN 2158-3188.
{{cite journal}}: Check date values in:|date=(help) - ^ Cerase, Andrea; Young, Alexander N.; Ruiz, Nerea Blanes; Buness, Andreas; Sant, Gabrielle M.; Arnold, Mirjam; Di Giacomo, Monica; Ascolani, Michela; Kumar, Manish; Hierholzer, Andreas; Trigiante, Giuseppe (15 April 2021). "Chd8 regulates X chromosome inactivation in mouse through fine-tuning control of Xist expression". Communications Biology. 4 (1). doi:10.1038/s42003-021-01945-1. ISSN 2399-3642.
- ^ Crespi BJ (30 June 2016). "Autism As a Disorder of High Intelligence". Frontiers in Neuroscience. 10: 300. doi:10.3389/fnins.2016.00300. PMC 4927579. PMID 27445671.
- ^ Baron-Cohen S (10 November 2020). The pattern seekers: how autism drives human invention. ISBN 978-1-5416-4713-8. OCLC 1204602315.
- ^ a b c d e f g h i j k Chen JA, Peñagarikano O, Belgard TG, Swarup V, Geschwind DH (2015). "The emerging picture of autism spectrum disorder: genetics and pathology". Annu Rev Pathol (Review). 10: 111–44. doi:10.1146/annurev-pathol-012414-040405. PMID 25621659.
- ^ Werling DM, Brand H, An JY, Stone MR, Zhu L, Glessner JT, et al. (April 2018). "An analytical framework for whole-genome sequence association studies and its implications for autism spectrum disorder". Nature Genetics. 50 (5): 727–736. doi:10.1038/s41588-018-0107-y. PMC 5961723. PMID 29700473.
- ^ Gardener H, Spiegelman D, Buka SL (August 2011). "Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis". Pediatrics. 128 (2): 344–55. doi:10.1542/peds.2010-1036. PMC 3387855. PMID 21746727.
- ^ a b Gardener H, Spiegelman D, Buka SL (July 2009). "Prenatal risk factors for autism: comprehensive meta-analysis". The British Journal of Psychiatry. 195 (1): 7–14. doi:10.1192/bjp.bp.108.051672. PMC 3712619. PMID 19567888.
- ^ Mazahery H, Camargo CA, Conlon C, Beck KL, Kruger MC, von Hurst PR (April 2016). "Vitamin D and Autism Spectrum Disorder: A Literature Review". Nutrients. 8 (4): 236. doi:10.3390/nu8040236. PMC 4848704. PMID 27110819.
- ^ Sarovic D (November 2021). "A Unifying Theory for Autism: The Pathogenetic Triad as a Theoretical Framework". Frontiers in Psychiatry (Review). 12: 767075. doi:10.3389/fpsyt.2021.767075. PMC 8637925. PMID 34867553. S2CID 244119594.
- ^ Markram K, Markram H (December 2010). "The Intense World Theory – a unifying theory of the neurobiology of autism". Frontiers in Human Neuroscience (Review). 4: 224. doi:10.3389/fnhum.2010.00224. PMC 3010743. PMID 21191475.
- ^ Deer B (8 February 2009). "MMR doctor Andrew Wakefield fixed data on autism". The Sunday Times.
- ^ Boseley S (2 February 2010). "Lancet retracts 'utterly false' MMR paper". The Guardian.
- ^ Stratton K, Ford A, Rusch E, Clayton EW, eds. (August 2011). "Influenza Vaccine". Adverse Effects of Vaccines: Evidence and Causality. Committee to Review Adverse Effects of Vaccines, Board on Population Health and Public Health Practice. Washington, D.C.: Institute of Medicine/National Academies Press. doi:10.17226/13164. ISBN 978-0-309-21435-3. PMID 24624471.
- ^ Flaherty DK (October 2011). "The vaccine-autism connection: a public health crisis caused by unethical medical practices and fraudulent science". The Annals of Pharmacotherapy. 45 (10): 1302–1304. doi:10.1345/aph.1Q318. PMID 21917556. S2CID 39479569.
- ^ Godlee F, Smith J, Marcovitch H (January 2011). "Wakefield's article linking MMR vaccine and autism was fraudulent". BMJ. 342: c7452. doi:10.1136/bmj.c7452. PMID 21209060. S2CID 43640126.
- ^ Tan M, Parkin JE (November 2000). "Route of decomposition of thiomersal (thimerosal)". International Journal of Pharmaceutics. 208 (1–2): 23–34. doi:10.1016/S0378-5173(00)00514-7. PMID 11064208.
- ^ a b Waterhouse L (December 2008). "Autism overflows: increasing prevalence and proliferating theories". Neuropsychology Review. 18 (4): 273–286. doi:10.1007/s11065-008-9074-x. PMID 19015994. S2CID 8863638.
- ^ "Frequently Asked Questions about Thimerosal". Centers for Disease Control and Prevention. Retrieved 21 February 2017.
- ^ Taylor LE, Swerdfeger AL, Eslick GD (June 2014). "Vaccines are not associated with autism: an evidence-based meta-analysis of case-control and cohort studies". Vaccine. 32 (29): 3623–3629. doi:10.1016/j.vaccine.2014.04.085. PMID 24814559.
- ^ Koenig K, Tsatsanis KD, Volkmar FR (2001). "Neurobiology and Genetics of Autism: A Developmental Perspective". In Burack JA, Charman T, Yirmiya N, Zelazo PR (eds.). The development of autism: perspectives from theory and research. Mahwah, N.J.: L. Erlbaum. pp. 73–92. ISBN 9780805832457. OCLC 806185029.
- ^ Minshew NJ (April 1996). "Brief report: brain mechanisms in autism: functional and structural abnormalities". Journal of Autism and Developmental Disorders. 26 (2): 205–9. doi:10.1007/BF02172013. PMID 8744486. S2CID 8134205.
- ^ Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM (June 2008). "Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies". European Psychiatry. 23 (4): 289–99. doi:10.1016/j.eurpsy.2007.05.006. PMID 17765485. S2CID 29070618.
- ^ Lefebvre A, Beggiato A, Bourgeron T, Toro R (July 2015). "Neuroanatomical Diversity of Corpus Callosum and Brain Volume in Autism: Meta-analysis, Analysis of the Autism Brain Imaging Data Exchange Project, and Simulation". Biological Psychiatry. 78 (2): 126–34. doi:10.1016/j.biopsych.2015.02.010. PMID 25850620. S2CID 8794474.
- ^ Sugranyes G, Kyriakopoulos M, Corrigall R, Taylor E, Frangou S (2011). "Autism spectrum disorders and schizophrenia: meta-analysis of the neural correlates of social cognition". PLOS ONE. 6 (10): e25322. Bibcode:2011PLoSO...625322S. doi:10.1371/journal.pone.0025322. PMC 3187762. PMID 21998649.
- ^ a b c d e O'Reilly C, Lewis JD, Elsabbagh M (2017). "Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies". PLOS ONE (Review). 12 (5): e0175870. Bibcode:2017PLoSO..1275870O. doi:10.1371/journal.pone.0175870. PMC 5414938. PMID 28467487.
- ^ a b c d e f g h Azhari A, Azizan F, Esposito G (July 2019). "A systematic review of gut-immune-brain mechanisms in Autism Spectrum Disorder". Developmental Psychobiology. 61 (s5): 752–771. doi:10.1002/dev.21803. hdl:10220/49107. PMID 30523646. S2CID 54523742.[better source needed]
- ^ Fadiga L, Craighero L, Olivier E (April 2005). "Human motor cortex excitability during the perception of others' action". Current Opinion in Neurobiology. 15 (2): 213–8. doi:10.1016/j.conb.2005.03.013. PMID 15831405. S2CID 10511430.
- ^ Shamay-Tsoory SG (February 2011). "The neural bases for empathy". The Neuroscientist. 17 (1): 18–24. doi:10.1177/1073858410379268. PMID 21071616. S2CID 2646438.
- ^ Dinstein I, Thomas C, Behrmann M, Heeger DJ (January 2008). "A mirror up to nature". Current Biology. 18 (1): R13-8. doi:10.1016/j.cub.2007.11.004. PMC 2517574. PMID 18177704.
- ^ Kalat J (2009). Biological Psychology (Tenth ed.). pp. 237–8. ISBN 978-0-495-60300-9.
- ^ Kennedy DP, Adolphs R (November 2012). "The social brain in psychiatric and neurological disorders". Trends in Cognitive Sciences. 16 (11): 559–72. doi:10.1016/j.tics.2012.09.006. PMC 3606817. PMID 23047070.
- ^ Schultz RT (2005). "Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area". International Journal of Developmental Neuroscience. 23 (2–3): 125–41. doi:10.1016/j.ijdevneu.2004.12.012. PMID 15749240. S2CID 17078137.
- ^ Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, Cohen BH (December 2007). "Mitochondrial disease: a practical approach for primary care physicians". Pediatrics. 120 (6): 1326–1333. doi:10.1542/peds.2007-0391. PMID 18055683. S2CID 4939996.
- ^ a b Rossignol DA, Frye RE (March 2012). "Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis". Molecular Psychiatry. 17 (3): 290–314. doi:10.1038/mp.2010.136. PMC 3285768. PMID 21263444.
- ^ Gentile S (August 2015). "Prenatal antidepressant exposure and the risk of autism spectrum disorders in children. Are we looking at the fall of Gods?". Journal of Affective Disorders. 182: 132–7. doi:10.1016/j.jad.2015.04.048. PMID 25985383.
- ^ Dragioti E, Solmi M, Favaro A, Fusar-Poli P, Dazzan P, Thompson T, et al. (October 2019). "Association of Antidepressant Use With Adverse Health Outcomes: A Systematic Umbrella Review". JAMA Psychiatry. 76 (12): 1241–1255. doi:10.1001/jamapsychiatry.2019.2859. PMC 6777224. PMID 31577342.
- ^ National Center on Birth Defects and Developmental Disabilities (13 March 2020). "Screening and Diagnosis: Autism Spectrum Disorder (ASD)". Centers for Disease Control and Prevention. US. Retrieved 21 September 2020.
- ^ Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A (June 2006). "Autism from 2 to 9 years of age". Archives of General Psychiatry. 63 (6): 694–701. doi:10.1001/archpsyc.63.6.694. PMID 16754843.
- ^ Mandell DS, Novak MM, Zubritsky CD (December 2005). "Factors associated with age of diagnosis among children with autism spectrum disorders". Pediatrics. 116 (6): 1480–1486. doi:10.1542/peds.2005-0185. PMC 2861294. PMID 16322174.
- ^ a b Volkmar F, Cook EH, Pomeroy J, Realmuto G, Tanguay P (December 1999). "Practice parameters for the assessment and treatment of children, adolescents, and adults with autism and other pervasive developmental disorders. American Academy of Child and Adolescent Psychiatry Working Group on Quality Issues". Journal of the American Academy of Child and Adolescent Psychiatry. 38 (12 Suppl): 32S–54S. doi:10.1016/s0890-8567(99)80003-3. PMID 10624084.
- ^ Constantino JN, Charman T (March 2016). "Diagnosis of autism spectrum disorder: reconciling the syndrome, its diverse origins, and variation in expression" (PDF). The Lancet. Neurology. 15 (3): 279–91. doi:10.1016/s1474-4422(15)00151-9. PMID 26497771. S2CID 206162618.
- ^ a b Simms MD (February 2017). "When Autistic Behavior Suggests a Disease Other than Classic Autism". Pediatric Clinics of North America. 64 (1): 127–138. doi:10.1016/j.pcl.2016.08.009. PMID 27894440.
- ^ Filipek PA, Accardo PJ, Ashwal S, Baranek GT, Cook EH, Dawson G, et al. (August 2000). "Practice parameter: screening and diagnosis of autism: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Child Neurology Society". Neurology. 55 (4): 468–79. doi:10.1212/wnl.55.4.468. PMID 10953176.
- ^ Filipek PA, Accardo PJ, Baranek GT, Cook EH, Dawson G, Gordon B, et al. (December 1999). "The screening and diagnosis of autistic spectrum disorders". Journal of Autism and Developmental Disorders. 29 (6): 439–84. doi:10.1023/A:1021943802493. PMID 10638459. S2CID 145113684.
- ^ a b Ozonoff S, Goodlin-Jones BL, Solomon M (September 2005). "Evidence-based assessment of autism spectrum disorders in children and adolescents" (PDF). Journal of Clinical Child and Adolescent Psychology. 34 (3): 523–40. doi:10.1207/s15374424jccp3403_8. PMID 16083393. S2CID 14322690.
- ^ Corsello C, Hus V, Pickles A, Risi S, Cook EH, Leventhal BL, Lord C (September 2007). "Between a ROC and a hard place: decision making and making decisions about using the SCQ". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 48 (9): 932–40. doi:10.1111/j.1469-7610.2007.01762.x. hdl:2027.42/74877. PMID 17714378.
- ^ Huerta M, Lord C (February 2012). "Diagnostic evaluation of autism spectrum disorders". Pediatric Clinics of North America. 59 (1): 103–11, xi. doi:10.1016/j.pcl.2011.10.018. PMC 3269006. PMID 22284796.
- ^ a b c d e f Siu AL, Bibbins-Domingo K, Grossman DC, Baumann LC, Davidson KW, Ebell M, et al. (February 2016). "Screening for Autism Spectrum Disorder in Young Children: US Preventive Services Task Force Recommendation Statement". JAMA. 315 (7): 691–696. doi:10.1001/jama.2016.0018. PMID 26881372.
- ^ a b c [non-primary source needed]Blumberg SJ, Zablotsky B, Avila RM, Colpe LJ, Pringle BA, Kogan MD (October 2016). "Diagnosis lost: Differences between children who had and who currently have an autism spectrum disorder diagnosis". Autism. 20 (7): 783–795. doi:10.1177/1362361315607724. PMC 4838550. PMID 26489772.
- ^ "Conditions That May Look Like Autism, but Aren't". WebMD. Retrieved 10 May 2020.
- ^ Helverschou SB, Bakken TL, Martinsen H (2011). Matson JL, Sturmey P (eds.). Psychiatric Disorders in People with Autism Spectrum Disorders: Phenomenology and Recognition. New York: Springer. pp. 53–74. ISBN 9781441980649. OCLC 746203105.
{{cite book}}:|work=ignored (help) - ^ Underwood L, McCarthy J, Tsakanikos E (September 2010). "Mental health of adults with autism spectrum disorders and intellectual disability". Current Opinion in Psychiatry. 23 (5): 421–6. doi:10.1097/YCO.0b013e32833cfc18. PMID 20613532. S2CID 13735841.
- ^ Ballaban-Gil K, Tuchman R (2000). "Epilepsy and epileptiform EEG: association with autism and language disorders". Developmental Disabilities Research Reviews. 6 (4): 300–8. doi:10.1002/1098-2779(2000)6:4<300::AID-MRDD9>3.0.CO;2-R. PMID 11107195.
- ^ Wiznitzer M (September 2004). "Autism and tuberous sclerosis". Journal of Child Neurology. 19 (9): 675–9. doi:10.1177/08830738040190090701. PMID 15563013. S2CID 38157900.
- ^ Zafeiriou DI, Ververi A, Vargiami E (June 2007). "Childhood autism and associated comorbidities". Brain & Development. 29 (5): 257–272. doi:10.1016/j.braindev.2006.09.003. PMID 17084999. S2CID 16386209.
- ^ Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, et al. (February 2020). "Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism". Cell. 180 (3): 568–584.e23. doi:10.1016/j.cell.2019.12.036. PMC 7250485. PMID 31981491.
- ^ "SYNGAP1-Related Intellectual Disability". Gene Reviews (Review). 2019. PMID 30789692.
- ^ O'Brien G, Pearson J (June 2004). "Autism and learning disability". Autism. 8 (2): 125–40. doi:10.1177/1362361304042718. PMID 15165430. S2CID 17372893.
- ^ Lainhart J (1999). "Psychiatric problems in individuals with autism, their parents and siblings". International Review of Psychiatry. 11 (4): 278–298. doi:10.1080/09540269974177.
- ^ Chisholm K, Lin A, Abu-Akel A, Wood SJ (August 2015). "The association between autism and schizophrenia spectrum disorders: A review of eight alternate models of co-occurrence" (PDF). Neuroscience and Biobehavioral Reviews. 55: 173–83. doi:10.1016/j.neubiorev.2015.04.012. PMID 25956249. S2CID 21450062.
- ^ Hamlyn J, Duhig M, McGrath J, Scott J (May 2013). "Modifiable risk factors for schizophrenia and autism—shared risk factors impacting on brain development". Neurobiology of Disease. 53: 3–9. doi:10.1016/j.nbd.2012.10.023. PMID 23123588. S2CID 207067275.
- ^ Crespi BJ, Thiselton DL (October 2011). "Comparative immunogenetics of autism and schizophrenia". Genes, Brain, and Behavior. 10 (7): 689–701. doi:10.1111/j.1601-183X.2011.00710.x. PMID 21649858. S2CID 851655.
- ^ Markkanen E, Meyer U, Dianov GL (June 2016). "DNA Damage and Repair in Schizophrenia and Autism: Implications for Cancer Comorbidity and Beyond". International Journal of Molecular Sciences. 17 (6): 856. doi:10.3390/ijms17060856. PMC 4926390. PMID 27258260.
- ^ Tsakanikos E, Costello H, Holt G, Sturmey P, Bouras N (July 2007). "Behaviour management problems as predictors of psychotropic medication and use of psychiatric services in adults with autism" (PDF). Journal of Autism and Developmental Disorders. 37 (6): 1080–5. doi:10.1007/s10803-006-0248-1. PMID 17053989. S2CID 14272598.
- ^ Rommelse NN, Franke B, Geurts HM, Hartman CA, Buitelaar JK (March 2010). "Shared heritability of attention-deficit/hyperactivity disorder and autism spectrum disorder". European Child & Adolescent Psychiatry. 19 (3): 281–95. doi:10.1007/s00787-010-0092-x. PMC 2839489. PMID 20148275.
- ^ Baranek GT (October 2002). "Efficacy of sensory and motor interventions for children with autism". Journal of Autism and Developmental Disorders. 32 (5): 397–422. doi:10.1023/A:1020541906063. PMID 12463517. S2CID 16449130.
- ^ a b Lugnegård T, Hallerbäck MU, Gillberg C (May 2012). "Personality disorders and autism spectrum disorders: what are the connections?". Comprehensive Psychiatry. 53 (4): 333–40. doi:10.1016/j.comppsych.2011.05.014. PMID 21821235.
- ^ Tantam D (December 1988). "Lifelong eccentricity and social isolation. II: Asperger's syndrome or schizoid personality disorder?". The British Journal of Psychiatry. 153: 783–91. doi:10.1192/bjp.153.6.783. PMID 3256377. S2CID 39433805.
- ^ Ekleberry SC (2008). "Cluster A - Schizoid Personality Disorder and Substance Use Disorders". Integrated Treatment for Co-Occurring Disorders: Personality Disorders and Addiction. Routledge. pp. 31–32. ISBN 978-0789036933.
- ^ McPartland J, Klin A (October 2006). "Asperger's syndrome". Adolescent Medicine Clinics. 17 (3): 771–88, abstract xiii. doi:10.1016/j.admecli.2006.06.010 (inactive 28 February 2022). PMID 17030291.
{{cite journal}}: CS1 maint: DOI inactive as of February 2022 (link) - ^ Woodbury-Smith MR, Volkmar FR (June 2008). "Asperger syndrome". European Child & Adolescent Psychiatry. 18 (1): 2–11. doi:10.1007/s00787-008-0701-0. PMID 18563474. S2CID 12808995.
- ^ Coplan J, Jawad AF (July 2005). "Modeling clinical outcome of children with autistic spectrum disorders". Pediatrics. 116 (1): 117–22. doi:10.1542/peds.2004-1118. PMID 15995041. S2CID 8440775.
- ^ "10 Facts about Autism Spectrum Disorder (ASD)". Early Childhood Development | ACF. Retrieved 6 November 2019.
- ^ Eldevik S, Hastings RP, Hughes JC, Jahr E, Eikeseth S, Cross S (May 2009). "Meta-analysis of Early Intensive Behavioral Intervention for children with autism". Journal of Clinical Child and Adolescent Psychology. 38 (3): 439–50. CiteSeerX 10.1.1.607.9620. doi:10.1080/15374410902851739. PMID 19437303. S2CID 205873629.
- ^ a b c d e Smith T, Iadarola S (2015). "Evidence Base Update for Autism Spectrum Disorder". Journal of Clinical Child and Adolescent Psychology. 44 (6): 897–922. doi:10.1080/15374416.2015.1077448. PMID 26430947.
- ^ a b c Myers SM, Johnson CP (November 2007). "Management of children with autism spectrum disorders". Pediatrics. 120 (5): 1162–82. doi:10.1542/peds.2007-2362. PMID 17967921. S2CID 1656920. Archived from the original on 12 June 2009.
- ^ Sigman M, Capps L (2002). Children with Autism: A Developmental Perspective. Cambridge: Harvard University Press. pp. 178–179. ISBN 978-0-674-05313-7.
- ^ Rogers SJ, Vismara LA (January 2008). "Evidence-based comprehensive treatments for early autism". Journal of Clinical Child and Adolescent Psychology. 37 (1): 8–38. doi:10.1080/15374410701817808. PMC 2943764. PMID 18444052.
- ^ Benevides TW, Shore SM, Andresen ML, Caplan R, Cook B, Gassner DL, et al. (August 2020). "Interventions to address health outcomes among autistic adults: A systematic review". Autism. 24 (6): 1345–1359. doi:10.1177/1362361320913664. PMC 7787674. PMID 32390461.
- ^ a b Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, et al. (October 2015). "Early Intervention for Children With Autism Spectrum Disorder Under 3 Years of Age: Recommendations for Practice and Research". Pediatrics. 136 (Supplement 1): S60-81. doi:10.1542/peds.2014-3667E. PMID 26430170.
- ^ Reichow B, Hume K, Barton EE, Boyd BA (May 2018). "Early intensive behavioral intervention (EIBI) for young children with autism spectrum disorders (ASD)". The Cochrane Database of Systematic Reviews. 5 (10): CD009260. doi:10.1002/14651858.CD009260.pub3. PMC 6494600. PMID 29742275.
- ^ Siewertsen CM, French ED, Teramoto M (2015). "Autism spectrum disorder and pet therapy". Advances in Mind-Body Medicine. 29 (2): 22–25. PMID 25831431.
- ^ a b Weissman L (March 2020). "Autism spectrum disorder in children and adolescents: Pharmacologic interventions". Retrieved 17 March 2020.
- ^ Williams Buckley A, Hirtz D, Oskoui M, et al. (March 2020). "Practice guideline: Treatment for insomnia and disrupted sleep behavior in children and adolescents with autism spectrum disorder: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology". Neurology. 94 (9): 392–404. doi:10.1212/WNL.0000000000009033. PMC 7238942. PMID 32051244.
- ^ Harrop C (August 2015). "Evidence-based, parent-mediated interventions for young children with autism spectrum disorder: The case of restricted and repetitive behaviors". Autism. 19 (6): 662–72. doi:10.1177/1362361314545685. PMID 25186943. S2CID 32326472.
- ^ Ameis SH, Kassee C, Corbett-Dick P, Cole L, Dadhwal S, Lai MC, et al. (November 2018). "Systematic review and guide to management of core and psychiatric symptoms in youth with autism". Acta Psychiatrica Scandinavica. 138 (5): 379–400. doi:10.1111/acps.12918. PMID 29904907. S2CID 49209337.
- ^ "Autism". World Health Organization. 30 March 2022. Retrieved 8 May 2022.
- ^ Fombonne E (June 2009). "Epidemiology of pervasive developmental disorders". Pediatric Research. 65 (6): 591–8. doi:10.1203/PDR.0b013e31819e7203. PMID 19218885.
- ^ Maenner MJ, Shaw KA, Baio J, Washington A, Patrick M, DiRienzo M, et al. (March 2020). "Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016". Morbidity and Mortality Weekly Report. Surveillance Summaries. 69 (4): 1–12. doi:10.15585/mmwr.ss6904a1. PMC 7119644. PMID 32214087.
- ^ "Autism spectrum disorder". ICD-11 for Mortality and Morbidity Statistics. International Classification of Diseases. World Health Organisation. February 2022 [2018; updates have since occurred]. 6A02. Retrieved 6 April 2022.
- ^ Volkmar FR, Lord C, Bailey A, Schultz RT, Klin A (January 2004). "Autism and pervasive developmental disorders". Journal of Child Psychology and Psychiatry, and Allied Disciplines. 45 (1): 135–70. doi:10.1046/j.0021-9630.2003.00317.x. PMID 14959806.
- ^ Tsakanikos E, Underwood L, Kravariti E, Bouras N, McCarthy J (2011). "Gender differences in co-morbid psychopathology and clinical management in adults with autism spectrum disorders". Research in Autism Spectrum Disorders. 5 (2): 803–808. doi:10.1016/j.rasd.2010.09.009. ISSN 1750-9467.
- ^ a b Newschaffer CJ, Croen LA, Daniels J, Giarelli E, Grether JK, Levy SE, et al. (2007). "The epidemiology of autism spectrum disorders". Annual Review of Public Health. 28: 235–58. doi:10.1146/annurev.publhealth.28.021406.144007. PMID 17367287.
- ^ Hollander E, Hagerman RJ, Fein D (30 April 2018). Autism spectrum disorders (First ed.). Washington, DC: American Psychiatric Association Publishing. ISBN 978-1-61537-192-1. OCLC 1022084798.
- ^ Baio J, Wiggins L, Christensen DL, et al. (April 2018). "Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years – Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014". Morbidity and Mortality Weekly Report. Surveillance Summaries. 67 (6): 1–23. doi:10.15585/mmwr.ss6706a1. PMC 5919599. PMID 29701730.
- ^ Rødgaard EM, Jensen K, Vergnes JN, Soulières I, Mottron L (August 2019). "Temporal Changes in Effect Sizes of Studies Comparing Individuals With and Without Autism: A Meta-analysis". JAMA Psychiatry. 76 (11): 1124–1132. doi:10.1001/jamapsychiatry.2019.1956. PMC 6704749. PMID 31433441.
- ^ "CDC Press Releases". CDC. 1 January 2016. Retrieved 31 December 2019.
- ^ Hill AP (2014). "Epidemiology of autism spectrum disorders". In Volkmar FR (ed.). Handbook of Autism and Pervasive Developmental Disorders. Diagnosis, Development, and Brain Mechanisms. Vol. 1. New York: Wiley. pp. 57–96. doi:10.1002/9781118911389. ISBN 9781118911389.
- ^ "HRSA-led study estimates 1 in 40 U.S. children has diagnosed autism". HRSA News Room. U.S. Department of Health & Human Services, Health Resources and Services Administration. 19 November 2018. Retrieved 17 October 2019.
- ^ National Center on Birth Defects and Developmental Disabilities (31 March 2022). "Autism and Developmental Disabilities Monitoring (ADDM) Network". Centers for Disease Control and Prevention. US. Retrieved 4 April 2022.
- ^ Murray S (9 September 2011). Autism (1st ed.). New York: Routledge. ISBN 978-0-415-88498-3. OCLC 773565341.
- ^ Kanner L (July 1949). "Problems of nosology and psychodynamics of early infantile autism". The American Journal of Orthopsychiatry. 19 (3): 416–26. doi:10.1111/j.1939-0025.1949.tb05441.x. PMID 18146742.
- ^ Tager-Flusberg H (February 2016). "Risk Factors Associated With Language in Autism Spectrum Disorder: Clues to Underlying Mechanisms". Journal of Speech, Language, and Hearing Research. 59 (1): 143–54. doi:10.1044/2015_jslhr-l-15-0146. PMC 4867927. PMID 26502110.
- ^ Harris J (February 2018). "Leo Kanner and autism: a 75-year perspective". International Review of Psychiatry. 30 (1): 3–17. doi:10.1080/09540261.2018.1455646. PMID 29667863. S2CID 4978549.
- ^ Aguiar MC, de Pondé MP (March 2019). "Parenting a child with autism". Jornal Brasileiro de Psiquiatria. 68 (1): 42–47. doi:10.1590/0047-2085000000223. ISSN 0047-2085. S2CID 165119472. Retrieved 17 February 2021.
- ^ Levinovitz A (29 April 2015). "An Alternative-Medicine Believer's Journey Back to Science". WIRED. Retrieved 13 February 2017.
The entire diagnosis and explanation took no more than 45 minutes. 'In the moment of diagnosis, it feels like the death of your hopes and dreams,' Louise [Laidler] says. There's a quiet grief in her voice, even though two decades have passed. 'In a way, it's even harder than a death, because you can't mourn and go on,' she says. 'You have to figure out how to care for your new child.'
- ^ Karst JS, Van Hecke AV (September 2012). "Parent and family impact of autism spectrum disorders: a review and proposed model for intervention evaluation". Clinical Child and Family Psychology Review. 15 (3): 247–77. doi:10.1007/s10567-012-0119-6. PMID 22869324. S2CID 19170894.
- ^ a b "The Autism Rights Movement". nymag.com. Retrieved 26 October 2021.
- ^ a b Ratner P (10 July 2016). "Should Autism Be Cured or Is "Curing" Offensive?". Big Think. Retrieved 16 June 2019.
- ^ a b Jaarsma P, Welin S (March 2012). "Autism as a natural human variation: reflections on the claims of the neurodiversity movement". Health Care Analysis. 20 (1): 20–30. doi:10.1007/s10728-011-0169-9. PMID 21311979. S2CID 18618887.
- ^ McGee M (August 2012). "Neurodiversity". Contexts. 11 (3): 12–13. doi:10.1177/1536504212456175. S2CID 220720495.
- ^ Sarrett J (April 2016). "Biocertification and Neurodiversity the Role and Implications of Self-Diagnosis in Autistic Communities". www.researchgate.net. Retrieved 6 March 2022.
- ^ Morgan J (1 October 2016). "Autism spectrum disorder: difference or disability?". The Lancet Neurology. 15 (11): 1126. doi:10.1016/S1474-4422(16)30002-3. ISSN 1474-4422. S2CID 54341655.
- ^ Silverman C (1 September 2008). "Fieldwork on Another Planet: Social Science Perspectives on the Autism Spectrum". BioSocieties. 3 (3): 325–341. doi:10.1017/S1745855208006236. ISSN 1745-8560. S2CID 145379758.
- ^ "A medical condition or just a difference? The question roils autism community". Washington Post. 3 May 2019. ISSN 0190-8286. Retrieved 15 October 2021.
- ^ a b c d e f g h Stichter JP, Riley-Tillman TC, Jimerson SR (December 2016). "Assessing, understanding, and supporting students with autism at school: Contemporary science, practice, and policy". School Psychology Quarterly. 31 (4): 443–449. doi:10.1037/spq0000184. PMID 27929316.
- ^ "Jean Piaget's Theory of Cognitive Development". Simply Psychology. Retrieved 19 February 2019.
- ^ "Facts and Statistics". Autism Society. Archived from the original on 30 October 2019. Retrieved 6 November 2019.
- ^ a b Ohl A, Grice Sheff M, Small S, Nguyen J, Paskor K, Zanjirian A (2017). "Predictors of employment status among adults with Autism Spectrum Disorder". Work. 56 (2): 345–355. doi:10.3233/WOR-172492. PMID 28211841.
- ^ Ganz ML (April 2007). "The lifetime distribution of the incremental societal costs of autism". Archives of Pediatrics & Adolescent Medicine. 161 (4): 343–349. doi:10.1001/archpedi.161.4.343. PMID 17404130.
- ^ Montes G, Halterman JS (April 2008). "Association of childhood autism spectrum disorders and loss of family income". Pediatrics. 121 (4): e821–e826. doi:10.1542/peds.2007-1594. PMID 18381511. S2CID 55179. Archived from the original on 4 March 2010.
- ^ Montes G, Halterman JS (July 2008). "Child care problems and employment among families with preschool-aged children with autism in the United States". Pediatrics. 122 (1): e202–e208. doi:10.1542/peds.2007-3037. PMID 18595965. S2CID 22686553. Archived from the original on 6 December 2009.
- ^ Johnson KR, Ennis-Cole D, Bohamgregory M (21 February 2020). "Workplace Success Strategies for Employees With Autism Spectrum Disorder: A New Frontier for Human Resource Development". Human Resource Development Review. 19 (2): 122–151. doi:10.1177/1534484320905910. S2CID 213995367.
- ^ Bernick M (12 January 2021). "The State Of Autism Employment In 2021". Forbes. Retrieved 11 February 2022.
