Eukaryotic DNA replication: Difference between revisions
→Cdc45 Protein: edited information |
→The Initiation Complex: added two kinases for triggering replication |
||
| Line 67: | Line 67: | ||
Binding of [[CDC45-related protein|Cdc45]] to [[chromatin]] depends on Clb-Cdc28 [[kinase]] activity as well as functional [[Cdc6|Cdc6]] and [[MCM2|Mcm2]], which suggests that [[CDC45-related protein|Cdc45]] associates with the [[pre-replication complex]] after activation of [[S-phase]] [[cyclin-dependent kinases]] (CDKs). As indicated by the timing and the [[Cyclin-dependent kinase|CDK]] dependence, binding of [[CDC45-related protein|Cdc45]] to chromatin is crucial for commitment to initiation of DNA replication. During S phase, [[CDC45-related protein|Cdc45]] physically interacts with [[minichromosome maintenance]] (MCM) proteins on chromatin; however, dissociation of [[CDC45-related protein|Cdc45]] from chromatin is slower than that of [[minichromosome maintenance|MCMs]], which indicates that the proteins are released by different mechanisms.<ref name="pmid9442876">{{cite journal |author=Dutta A, Bell SP |title=Initiation of DNA replication in eukaryotic cells |journal=Annu. Rev. Cell Dev. Biol. |volume=13 |issue= |pages=293–332 |year=1997 |pmid=9442876 |doi=10.1146/annurev.cellbio.13.1.293 |url=}}</ref> |
Binding of [[CDC45-related protein|Cdc45]] to [[chromatin]] depends on Clb-Cdc28 [[kinase]] activity as well as functional [[Cdc6|Cdc6]] and [[MCM2|Mcm2]], which suggests that [[CDC45-related protein|Cdc45]] associates with the [[pre-replication complex]] after activation of [[S-phase]] [[cyclin-dependent kinases]] (CDKs). As indicated by the timing and the [[Cyclin-dependent kinase|CDK]] dependence, binding of [[CDC45-related protein|Cdc45]] to chromatin is crucial for commitment to initiation of DNA replication. During S phase, [[CDC45-related protein|Cdc45]] physically interacts with [[minichromosome maintenance]] (MCM) proteins on chromatin; however, dissociation of [[CDC45-related protein|Cdc45]] from chromatin is slower than that of [[minichromosome maintenance|MCMs]], which indicates that the proteins are released by different mechanisms.<ref name="pmid9442876">{{cite journal |author=Dutta A, Bell SP |title=Initiation of DNA replication in eukaryotic cells |journal=Annu. Rev. Cell Dev. Biol. |volume=13 |issue= |pages=293–332 |year=1997 |pmid=9442876 |doi=10.1146/annurev.cellbio.13.1.293 |url=}}</ref> |
||
====Two Protein Kinases Triggering DNA Replication==== |
|||
At the onset of the [[S phase]], the [[Pre-replication complex|pre-replicative complex]] must be activated by two [[S phase]] specific [[kinases]] in order to form an initiation complex at an [[origin of replication]]. One [[kinase]] is the Cdc7-Dbf4 kinase called Dbf4-dependent kinase (DDK) and the other is [[cyclin-dependent kinase]] (CDK).<ref name="pmid10577390">{{cite journal |author=Masai H, Sato N, Takeda T, Arai K |title=CDC7 kinase complex as a molecular switch for DNA replication |journal=Front. Biosci. |volume=4 |issue= |pages=D834–40 |year=1999 |month=December |pmid=10577390 |doi= |url=}}</ref> Chromatin-binding assays of [[CDC45-related protein|Cdc45]] in [[yeast]] and [[Xenopus|''Xenopus'']] have shown that a downstream event of [[cyclin-dependent kinase|CDK]] action is loading of [[CDC45-related protein|Cdc45]] onto chromatin. <ref name=pmid9755170 /><ref name=pmid9554851 /> [[Cdc6]] has been speculated to be a target of [[cyclin-dependent kinase|CDK]] action, because of the association between [[Cdc6]] and [[cyclin-dependent kinase|CDK]], and the CDK-dependent [[phosphorylation]] of [[Cdc6]]. The CDK-dependent [[phosphorylation]] of [[Cdc6]] has been considered to be required for entry into the [[S phase]].<ref name="pmid10339564">{{cite journal |author=Jiang W, Wells NJ, Hunter T |title=Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6 |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=96 |issue=11 |pages=6193–8 |year=1999 |month=May |pmid=10339564 |pmc=26858 |doi= |url=}}</ref> |
|||
Both the [[catalytic]] subunit of DDK, Cdc7, and the activator protein, Dbf4, are conserved in eukaryotes and are required for the onset of the [[S phase]] of the [[cell cycle]].<ref name="pmid10523313">{{cite journal |author=Jiang W, McDonald D, Hope TJ, Hunter T |title=Mammalian Cdc7-Dbf4 protein [[kinase]] complex is essential for initiation of [[DNA replication]] |journal=EMBO J. |volume=18 |issue=20 |pages=5703–13 |year=1999 |month=October |pmid=10523313 |pmc=1171637 |doi=10.1093/emboj/18.20.5703 |url=}}</ref><ref name="pmid10373557">{{cite journal |author=Kumagai H, Sato N, Yamada M, ''et al.'' |title=A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells |journal=Mol. Cell. Biol. |volume=19 |issue=7 |pages=5083–95 |year=1999 |month=July |pmid=10373557 |pmc=84351 |doi= |url=}}</ref> Both DDK and Cdc7 are required for the loading of [[CDC45-related protein|Cdc45]] onto [[chromatin]] [[origins of replication]]. The target for binging of the DDK [[kinase]] is the [[Minichromosome maintenance|MCM complex]], possibly [[MCM2|Mcm2]].<ref name=pmid10523313 /> DDK targets the [[Minichromosome maintenance|MCM complex]] and its phosphorylation leads to the possible activation of [[Minichromosome maintenance|MCM]] [[helicase]] activity.<ref name="pmid9096361">{{cite journal |author=Hardy CF, Dryga O, Seematter S, Pahl PM, Sclafani RA |title=mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p |journal=Proc. Natl. Acad. Sci. U.S.A. |volume=94 |issue=7 |pages=3151–5 |year=1997 |month=April |pmid=9096361 |pmc=20337 |doi= |url=}}</ref> |
|||
==Elongation== |
==Elongation== |
||
Revision as of 17:20, 25 April 2013

The process of eukaryotic DNA replication is a conserved mechanism that restricts DNA replication to only once per cell cycle. Eukaryotic DNA replication of chromosomal DNA is central for the duplication of a cell and is necessary for the maintenance of the eukaryotic genome.
When compared to prokaryotic DNA replication, the completion of eukaryotic DNA replication is more difficult and involves multiple origins of replication and replicative proteins to accomplish. Prokaryotic DNA is arranged in a circular shape, and has only one replication origin when replication starts. By contrast, eukaryotic DNA is linear. When replicated, there are as many as one thousand origins of replication.
Eukaryotic DNA replication requires precise coordination of all DNA polymerases and associated proteins to replicate the entire genome each time a cell divides. This process is achieved through a series of steps of protein assemblies at origins of replication. These origins of replication direct the number of protein complexes that will form to initiate replication during the G1 stage of the cell life cycle. Despite these differences, however, the underlying process of replication is the same for both prokaryotic and eukaryotic DNA.
Initiation of Replication
DNA replication is initiated from specific sequences called replication origins. Eukaryotic cells have multiple replication origins. To initiate DNA replication, many proteins assemble on and dissociate from these origins of replication.[1] The individual factors described below work together to direct the formation of the pre-replication complex (pre-RC), a key intermediate in the replication initiation process. Association of the origin recognition complex (ORC) with the origin of replication is required to recruit both Cell Division Cycle 6 protein (Cdc6p) and Chromatin licensing and DNA replication factor 1 protein (Cdt1p), which initiates the assembly of the pre-RC. Both Cdc6p and Cdt1p associate with the ORC bound to chromatin independently from each other. ORC, Cdc6p, and Cdt1p together are required for the stable association of the Minichromosome Maintenance (Mcm 2-7) Complex proteins with the origin during G1 phase of the cell cycle.[2]
The Pre-replicative Complex
- Main article: Pre-replication complex
Eukaryotic origins of replication control the formation of a number of protein complexes that lead to the assembly of two bidirectional DNA replication forks. These events are initiated by the formation of the pre-replication complex (pre-RC) at the origins of replication. This process takes place in the G1 phase of the cell cycle. The pre-RC formation involves the ordered assembly of many replication factors including the Origin Recognition Complex (ORC), Cdc6 protein, Cdt1 protein, and Minichromosome maintenance proteins (Mcm2-7p).[3] Once the pre-RC is formed, activation of the complex is triggered by two kinases, cyclin-dependent kinase (CDK) and Dbf4-dependent kinase (Ddk) that help transition to DNA replication. This transition involves the ordered assembly of additional replication factors to unwind the DNA and accumulate the multiple eukaryotic DNA polymerases around the unwound DNA.
The Origin Recognition Complex
- Main article: Origin recognition complex

The Origin Recognition Complex (ORC) is a six-subunit, ORC 1p-6p, protein complex that selects the sites on DNA for initiation of replication at eukaryotic origins of replication.[4] ORC binding to chromatin is regulated through the cell cycle.[3] Generally, the function and size of the ORC subunits are conserved throughout many eukaryotic genomes with the difference being which ORC unit actually contacts the DNA.[5]
The most widely studied ORC is that of Saccharomyces cerevisiae or yeast which is known to bind to the autonomously replicating sequence (ARS). The ORC of higher eukaryotes are made up of similar AT rich regions.[6] The S. cerevisiae ORC interacts specifically with both the A and B1 elements of yeast origins of replication, spanning a region of 30 base pairs. The binding to these sequences requires ATP.[3] The S. cerevisiae ORC requires the five largest subunits, Orc1, Orc2, Orc3, Orc4, and Orc5, to recognize DNA four of which (Ocr1p, 2p, 4p, and 5p) are in close contact with the origin.[3] The Orc1p and Orc5p subunits are known to interact with ATP, but only the interaction between the Orc1p subunit and ATP are required for DNA binding.[7] The S. cerevisiae ORC also hydrolyzes ATP and this also depends on the Ocr1p subunit; however ATP hydrolysis is not required for DNA binding.[3] The ORC needs to be in an ATP-bound state so that it can interact with Cdc6p creating events that will produce the unwinding of the double stranded DNA which will promote the hydrolysis of the ATP by the ORC.[3] Once the ORC is bound to the origin, the complex is retained in an ATP-bound state and the ATP hydrolysis is reserved for a downstream step in initiation. When the ORC binds to DNA at replication origin sites in an ATP‐dependent manner it then serves as a scaffold for the assembly of other key initiation factors of the pre-replicative complex (pre-RC) which includes Cdc6, Cdt1, and Minichromosome Maintenance (Mcm 2-7) Complex proteins.[8]
This pre-RC assembly during the G1 phase of the cell cycle is required prior to the continuation of DNA replication during the S phase.[9] The regulation of mammalian ORC is consistent with the removal of at least part of the complex from the chromosome at metaphase. The Orc1p associated with chromatin is released during mitosis. The removal of ORC could serve to eliminate the pre-RC formation prior to the completion of metaphase.[10]
Cdc6 Protein
- Main article: Cdc6

Binding of the cell division cycle 6 (Cdc6) protein to the Origin Recognition Complex (ORC) is a key step in the assembly of the pre-replication complex (pre-RC) at the origins of replication. The Cdc6p will bind to the ORC on DNA in an ATP-dependent manner, which will induce a change in the pattern of origin binding that requires Orc10 ATPase.[7] The Cdc6p requires ORC to associate with chromatin and is in turn required for Mcm2-7p to bind to the chromatin.[11] The ORC-Cdc6p complex forms a ring-shaped structure and is analogous to other ATP-dependent protein machines. The binding of Cdc6p to ORC is essential for the assembly of the pre-RC in which the levels and activity of Cdc6 regulate the frequency with which origin of replication is utilized during the cell cycle.
Cdt1 Protein
- Main article: Cdt1
In fission yeast and Xenopus, chromatin licensing and DNA replication factor 1 (Cdt1) protein is required for the licensing of chromatin for DNA replication. Cdt1 is essential for DNA replication and performs it role during the formation of the pre-replicative complex by loading the minichromosome maintenance proteins onto the chromosome.[12] Cdt1p has been shown to associate with the C terminus of the Cdc6p to cooperatively promote the association of Mcm proteins to the chromatin.[13] Cdt1p activity during the cell cycle is tightly regulated by its association with the protein geminin, which both inhibits Cdt1 activity during S phase in order to prevent re-replication of DNA and prevents it from ubiquitination and subsequent proteolysis.[14]
Minichromosome Maintenance (Mcm 2-7) Protein Complex
- Main article: Minichromosome maintenance Proteins

The assembly of the minichromosome maintenance proteins (Mcm) function together as a complex in the cell. The assembly of the Mcm proteins onto the chromatin requires the coordinated function of the Origin Recognition Complex, Cdc6p, and Cdt1p.[15] Once the Mcm proteins have been loaded onto the chromatin, ORC and Cdc6p can be removed from the chromatin without preventing subsequent DNA replication. This suggests that the primary role of the pre-RC is to correctly load the Mcm proteins.[16] The Mcm proteins support roles both in the initiation and elongation steps of DNA synthesis.[17] Each Mcm proteins is highly related to all others, but unique sequences distinguishing each of the subunit types are conserved across eukaryotes. All eukaryotes have exactly six Mcm proteins analogs that each fall into one of the existing classes (Mcm2-7p), which suggests that each Mcm proteins has a unique and important function.[18]
Mcm proteins have been found to be required for DNA helicase activity and inactivation of any of the six Mcm proteins prevents further progression of the replication fork. This is consistent with the requirement of ORC, Cdc6p, and Cdt1p function to assemble the Mcm proteins at the origin.[19] The structure of the Mcm proteins support a hexameric structure for the Mcm complex. The complex containing all six Mcm proteins creates a doughnut like structure with a central cavity.[20] The helicase activity of the Mcm protein complex raised an interesting question of how theMcm proteins ring-like complex is loaded onto the single-stranded DNA. One possibility is that the helicase activity of the Mcm protein complex can oscillate between an open and a closed ring formation to allow single-stranded DNA loading.[3]
Along with the Mcm protein complex helicase activity, the complex also has associated ATPase activity.[21] A mutation in any one of the six Mcm proteins reduces the conserved ATP binding sites, which indicates that ATP hydrolysis is a coordinated event involving all six subunits of the Mcm complex.[22] Studies have shown that within the Mcm protein complexare specific catalytic pairs of Mcm proteins that function together to coordinate ATP hydrolysis. For example, Mcm3p but not Mcm6p can activate Mcm6p activity. These studies suggest that the structure for the Mcm hexamer with Mcm3p next to Mcm7p, Mcm2p next to Mcm6p, and Mcm4p next to Mcm5p. Both members of the catalytic pairs contribute to the conformation that allows ATP binding and hydrolysis. The mixture of active and inactive subunits create a coordinated ATPase activity that allows the Mcm protein complex to complete ATP binding and hydrolysis as a whole.[23]
The nuclear localization of Mcm proteins is regulated in budding yeast cells. The Mcm proteins are present in the nucleus in G1 and S phase of the cell cycle, but are exported to the cytoplasm during the G2 and M phase. The complete, intact six subunit Mcm complex is required to enter into the nucleus.[24] In S. cerevisiae, nuclear export is promoted by cyclin-dependent kinases (CDK) activity. Mcm proteins that are associated with chromatin are protected from CDK export machinery due to the lack of accessibility to CDK.[25]
The Initiation Complex
In addition to the binding of the Minichromosome maintenance proteins (Mcm), Cell division cycle 45 (Cdc45) protein is also essential for initiating DNA replication.[26][27] Studies have shown that Mcm is critical for the loading of Cdc45 onto chromatin and this complex containing both Mcm and Cdc45 is formed at the onset of the S phase of the cell cycle.[28][29] Cdc45 targets Mcm protein complex, which has been loaded onto the chromatin as a component of the pre-replicative complex at the origin of replication during the G1 phase of the cell cycle.[17]
Cdc45 Protein
- Main article: Cdc45
Cell division cycle 45 (Cdc45) protein is a critical component for the conversion of the pre-replicative complex to the initiation complex. The Cdc45 protein assembles at replication origins before initiation and is required for replication to begin at replication origins in Saccharomyces cerevisiae and has an essential role during elongation. Thus, Cdc45 has central roles in both initiation and elongation phases of chromosomal DNA replication.[30] Cdc45 is a replicator factor that physically associates with Mcm5p and displays genetic interactions with five of the six members of the Mcm gene family and ORC2 gene and Cdc45p is also a component of the pre-replicative complex.[17]
Cdc45, a protein for initiation of DNA replication, associates with chromatin after 'start' in late G1 phase and during the S phase of the cell cycle. The loading of Cdc45 onto chromatin is critical for loading various replication proteins, including DNA polymerase α, DNA polymerase ε, replication protein A (RPA) and proliferating cell nuclear antigen (PCNA) onto chromatin. [29][31][32][33] Within a Xenopus nucleus-free system, it has been demonstrated that Cdc45 is required for the unwinding of plasmid DNA.[33] The Xenopus nucleus-free system also demonstrates that DNA unwinding and tight RPA binding to chromatin occurs only in the presence of Cdc45.[29]
Binding of Cdc45 to chromatin depends on Clb-Cdc28 kinase activity as well as functional Cdc6 and Mcm2, which suggests that Cdc45 associates with the pre-replication complex after activation of S-phase cyclin-dependent kinases (CDKs). As indicated by the timing and the CDK dependence, binding of Cdc45 to chromatin is crucial for commitment to initiation of DNA replication. During S phase, Cdc45 physically interacts with minichromosome maintenance (MCM) proteins on chromatin; however, dissociation of Cdc45 from chromatin is slower than that of MCMs, which indicates that the proteins are released by different mechanisms.[18]
Two Protein Kinases Triggering DNA Replication
At the onset of the S phase, the pre-replicative complex must be activated by two S phase specific kinases in order to form an initiation complex at an origin of replication. One kinase is the Cdc7-Dbf4 kinase called Dbf4-dependent kinase (DDK) and the other is cyclin-dependent kinase (CDK).[34] Chromatin-binding assays of Cdc45 in yeast and Xenopus have shown that a downstream event of CDK action is loading of Cdc45 onto chromatin. [27][28] Cdc6 has been speculated to be a target of CDK action, because of the association between Cdc6 and CDK, and the CDK-dependent phosphorylation of Cdc6. The CDK-dependent phosphorylation of Cdc6 has been considered to be required for entry into the S phase.[35]
Both the catalytic subunit of DDK, Cdc7, and the activator protein, Dbf4, are conserved in eukaryotes and are required for the onset of the S phase of the cell cycle.[36][37] Both DDK and Cdc7 are required for the loading of Cdc45 onto chromatin origins of replication. The target for binging of the DDK kinase is the MCM complex, possibly Mcm2.[36] DDK targets the MCM complex and its phosphorylation leads to the possible activation of MCM helicase activity.[38]
Elongation
The formation of the pre-replicative complex (pre-RC) marks the potential sites for the initiation of DNA replication. Consistent with the Minichromosome maintenance (Mcm2-7) complex encircling double stranded DNA, formation of the pre-RC does not lead to the immediate unwinding of origin DNA or the recruitment of DNA polymerases. Instead, the pre-RC that is formed during the G1 of the cell cycle is only activated to unwind the DNA and initiate replication after the cells pass from the G1 phase to the S phase of the cell cycle.[39] There are multiple other proteins or protein complexes that must associate with the origin prior to a successful initiation of DNA synthesis. These proteins include regulatory factors as well as components of the DNA replication fork. DNA helicases, sliding clamps, single stranded binding proteins, primase, DNA polymerases, topoisomerases, RNAse H and DNA ligase all play an important role in elongation at the replication fork.
DNA helicases couple ATP hydrolysis with separation of DNA strands that will form the replication fork. The Mcm2-7 complex, a ring-shaped heterohexameric ATPase, is the best candidate for the replicative DNA helicase.[40] In yeast, five out of the six Mcm2-7 subunits are required for replication fork progression, and chromatin immunoprecipitation (ChIP) experiments indicate that Mcm4 and Mcm7 travel with the DNA replication fork.[19] [17] Besides Mcm2-7, there are several other factors that have essential roles in origin unwinding and elongation and therefore may function as replicative helicase cofactors. Cdc45 is an essential component of the replication fork.[17][41] It has been shown that antibodies against Cdc45 block DNA unwinding during replication in Xenopus egg extracts. [41] Also, Cdc45 has been coimmunoprecipitated with Mcm proteins as well as DNA helicase activity from a chromatin fraction.[42][43][28]
Once the DNA strands have been separated by the DNA helicases, single stranded binding proteins bind and stabilize the single-stranded DNA (ssDNA) strands. As the DNA is unwound the twist number decreases. To compensate for this the writhe number increases, introducing positive supercoils in the DNA. These supercoils would cause DNA replication to halt if they were not removed. Topoisomerases are responsible for removing these supercoils ahead of the replication fork.
Replication Fork
The replication fork is the junction the between the newly separated template strands, known as the leading and lagging strands, and the double stranded DNA. The replication fork continually moves in the direction of the double stranded DNA. Due to the fact that DNA polymerase can only add nucleotides to the 3' end of DNA, the leading strand is continuously synthesized towards the replication fork while the lagging strand is discontinuously synthesized away from the replication fork.
Leading Strand
Once an RNA primer has been added, by primase, to the 5' end of the leading strand, DNA synthesis will continue in a 3' to 5' direction uninterrupted. Therefore leading strand synthesis requires only one primer with uninterrupted DNA polymerase activity.
Lagging Strand
In lagging strand synthesis, the movement of the DNA polymerase in the opposite direction of the replication fork requires the use of multiple RNA primers. DNA polymerase will synthesize short fragments of DNA called Okazaki fragments which are added to the 3' end of the primer. These fragments can be anywhere between 100-400 nucleotides long in eukaryotes.[44]
Primase is responsible for creating short RNA primers that are added to the single-stranded DNA (ssDNA) template. These primers are extended by DNA polymerases. At least three different types of eukaryotic DNA polymerases are involved in the replication of DNA in eukaryotic cells (POL α, Pol δ and POL ε).
- Pol α forms a complex with a small catalytic (PriS) and a large noncatalytic (PriL) subunit,[45] with the Pri subunits acting as a primase (synthesizing an RNA primer), and then with DNA Pol α elongating that primer with DNA nucleotides. After around 20 nucleotides[46] elongation is taken over by Pol ε (on the leading strand) and δ (on the lagging strand).
- Pol δ: Highly processive and has proofreading 3'->5' exonuclease activity. Thought to be the main polymerase involved in lagging strand synthesis, though there is still debate about its role.[47]
- Pol ε: Also highly processive and has proofreading 3'->5' exonuclease activity. Highly related to pol δ, and thought to be the main polymerase involved in leading strand synthesis,[48] though there is again still debate about its role.[47]
RNAse H recognizes the DNA:RNA hybrids that are created by the use of RNA primers and is responsible for removing these from the replicated strand, leaving behind a primer:template junction. DNA polymerase, most likely Pol α, recognizes these sites and elongates the breaks left by primer removal. Replication of the lagging strand requires a final enzyme. DNA ligase then repairs the nicks that are left between the 3'-OH and 5'phosphate of the newly synthesized strand. DNA synthesis is complete once all RNA primers are removed and nick are repaired.
Termination of Replication
Termination of eukaryotic DNA replication requires different processes depending on whether the chromosomes are circular or linear. Unlike linear molecules, circular chromosomes are able to replicate the entire molecule. However, the two DNA molecules will remain linked together. This issue is handled by decantenation of the two DNA molecules by a type II topoisomerase. Type II topoisomerases are also used to separate linear strands as they are intricately folded into a nucleosome within the cell.
As previously mentioned linear chromosomes face another issue that is not seen in circular DNA replication. Due to the fact that an RNA primer is required for initiation of DNA synthesis the lagging strand is at a disadvantage in replicating the entire chromosome. While the leading strand can use a single RNA primer to extend the 5' terminus of the replicating DNA strand, multiple RNA primers are responsible for lagging strand synthesis creating Okazaki fragments. This leads to an issue due to the fact that DNA polymerase is only able to add to the 3' end of the DNA strand. The 3'-5' action of DNA polymerase will leave a short single-stranded DNA (ssDNA) region at the 5' end of the replicated DNA when the Okazaki fragments have been repaired. Over time this would result in progressive shortening of one of the daughter chromosomes. This is known as the end replication problem.
The end replication problem is handled in eukaryotic cells by telomere regions and telomerase. Telomeres extend the 3' end of the parental chromosome beyond the 5' end of the daughter strand. This ssDNA structure can act as an origin of replication that recruits telomerase. Telomerase is a specialized DNA polymerase that consists of multiple protein subunits and an RNA component. The RNA component of telomerase anneals to the single stranded 3' end of the template DNA and contains 1.5 copies of the telomeric sequence.[44] Telomerase contains a protein subunit that is a reverse transcriptase called telomerase reverse transcriptase or TERT. TERT synthesizes DNA until the end of the template telomerase RNA and then disengages.[44] This process can be repeated as many times as needed with the extension of the 3' end of the parental DNA molecule. This 3' addition provides a template for extension of the 5' end of the daughter strand by lagging strand DNA synthesis. Regulation of telomerase activity is handled by telomere-binding proteins.
Regulation of Replication
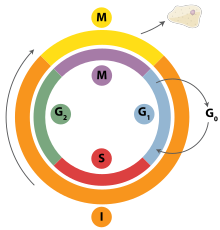
DNA replication is a tightly orchestrated process that is controlled within the context of the cell cycle. Progress through the cell cycle and in turn DNA replication is tightly regulated by the formation and activation of pre-replicative complexs (pre-RCs) which is achieved through the activation and inactivation of cyclin-dependent kinases (Cdks). Specifically it is the interactions of cyclins and cyclin dependent kinases that are responsible for the transition from G1 into S-phase.
During the G1 phase of the cell cycle there are low levels of Cdk activity. This low level of Cdk activity allows for the formation of new pre-RC complexes but is not sufficient for DNA replication to be initiated by the newly formed Pre-replication complex|pre-RCs]]. During the remaining phases of the cell cycle there are elevated levels of Cdk activity. This high level of Cdk activity is responsible for initiating DNA replication as well as inhibiting new pre-RC complex formation.[39] Once DNA replication has been initiated the pre-RC complex is broken down. Due to the fact that Cdk levels remain high during the S, G2, and M phases of the cell cycle no new pre-RC complexes can be formed. This all helps to ensure that no initiation can occur until the cell division is complete.
In addition to cyclin dependent kinases a new round of replication is thought to be prevented through the downregulation of Cdt1. This is acheived via degradation of Cdt1 as well as through the inhibitory actions of a protein known as geminin. Geminin binds tightly to Cdt1 and is tought to be the major inhibitor of re-replication.[39] Geminin first appears in S-phase and is degraded at the metaphase-anaphase transition, possibly through ubiquination by anaphase promoting complex (APC).[49]
Various cell cycle checkpoints are present throughout the course of the cell cycle that determine whether a cell will progress through division entirely. Importantly in replication the G1, or restriction, checkpoint makes the determination of whether or not initiation of replication will begin or whether the cell will be placed in a resting stage known as G0. Cells in the G0 stage of the cell cycle are prevented from initiating a round of replication because the Mcm proteins are not expressed. Transition into the S-phase indicates replication has begun.
Eukaryotic DNA Replication Proteins
List of major proteins involved in Eukaryotic DNA replication
| Protein | Function in Eukaryotic DNA replication |
|---|---|
| Origin Recognition Complex | Heterohexameric complex composed of Orc1-6. Binds to DNA and assembles Mcm2-7 complex onto chromatin (also requires Cdc6 and Cdt1). |
| Cdc6 | Required for assembly of Mcm2-7 complex at ORC, in conjunction with Cdt1 . |
| Cdt1 | Loads Mcm2-7 complex on DNA at at ORC in pre-RC/licensing step. Inhibited by geminin. |
| Mcm2-7 | Assembled onto chromatin at ORC to form pre-replicative complex. On replication initiation, Mcm2-7 moves away from ORC with replication fork. Probably provides helicase activity at fork. |
| Cdc45 | Required for initiation and elongation steps of DNA replication. May be part of Mcm2-7 helicase complex.
Required after pre-RC step for loading of various proteins for initiation and elongation. |
| Dpb11 | Required for initiation of DNA replication. |
| GINS | Tetrameric complex composed of Sld5, Psf1, Psf2, Psf3. Associates with pre-replicative complex around the time of initiation and moves with replication forks during elongation step. Required for elongation stage of DNA replication and maybe part of the Mcm helicase complex. |
| DNA Polymerase α | Hetero-tetrameric polymerase which has intrinsic DNA primase activity. Required for initial DNA synthesis of leading strand at origins and for priming of Okazaki fragments on lagging strand. |
| DNA Polymerase δ | Replicative polymerase; synthesizes DNA at the replication fork. May function on the lagging strand. |
| DNA Polymerase ε | Replicative polymerase; synthesizes DNA at the replication fork. May function on the leading strand. |
See Also
References
- ^ Araki H (2011). "Initiation of chromosomal DNA replication in eukaryotic cells; contribution of yeast genetics to the elucidation". Genes Genet. Syst. 86 (3): 141–9. PMID 21952204.
- ^ Maiorano D, Moreau J, Méchali M (2000). "XCDT1 is required for the assembly of pre-replicative complexes in Xenopus laevis". Nature. 404 (6778): 622–5. doi:10.1038/35007104. PMID 10766247.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Bell SP, Dutta A (2002). "DNA replication in eukaryotic cells". Annu. Rev. Biochem. 71: 333–74. doi:10.1146/annurev.biochem.71.110601.135425. PMID 12045100.
- ^ Bell SP (2002). "The origin recognition complex: from simple origins to complex functions". Genes Dev. 16 (6): 659–72. doi:10.1101/gad.969602. PMID 11914271.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bielinsky AK, Gerbi SA (2001). "Where it all starts: eukaryotic origins of DNA replication". J. Cell. Sci. 114 (Pt 4): 643–51. PMID 11171369.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Xu W, Aparicio JG, Aparicio OM, Tavaré S (2006). "Genome-wide mapping of ORC and Mcm2p binding sites on tiling arrays and identification of essential ARS consensus sequences in S. cerevisiae". BMC Genomics. 7: 276. doi:10.1186/1471-2164-7-276. PMC 1657020. PMID 17067396.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b Speck C, Chen Z, Li H, Stillman B (2005). "ATPase-dependent cooperative binding of ORC and Cdc6 to origin DNA". Nat. Struct. Mol. Biol. 12 (11): 965–71. doi:10.1038/nsmb1002. PMC 2952294. PMID 16228006.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chesnokov IN (2007). "Multiple functions of the origin recognition complex". Int. Rev. Cytol. 256: 69–109. doi:10.1016/S0074-7696(07)56003-1. PMID 17241905.
- ^ Matsuda K, Makise M, Sueyasu Y, Takehara M, Asano T, Mizushima T (2007). "Yeast two-hybrid analysis of the ORC of Saccharomyces cerevisiae: interaction between subunits and identification of binding proteins". FEMS Yeast Res. 7 (8): 1263–9. doi:10.1111/j.1567-1364.2007.00298.x. PMID 17825065.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kreitz S, Ritzi M, Baack M, Knippers R (2001). "The human origin recognition complex protein 1 dissociates from chromatin during S phase in HeLa cells". J. Biol. Chem. 276 (9): 6337–42. doi:10.1074/jbc.M009473200. PMID 11102449.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Coleman TR, Carpenter PB, Dunphy WG (1996). "The Xenopus Cdc6 protein is essential for the initiation of a single round of DNA replication in cell-free extracts". Cell. 87 (1): 53–63. PMID 8858148.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rialland M, Sola F, Santocanale C (2002). "Essential role of human CDT1 in DNA replication and chromatin licensing". J. Cell. Sci. 115 (Pt 7): 1435–40. PMID 11896191.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nishitani H, Lygerou Z, Nishimoto T, Nurse P (2000). "The Cdt1 protein is required to license DNA for replication in fission yeast". Nature. 404 (6778): 625–8. doi:10.1038/35007110. PMID 10766248.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A (2000). "Inhibition of eukaryotic DNA replication by geminin binding to Cdt1". Science. 290 (5500): 2309–12. doi:10.1126/science.290.5500.2309. PMID 11125146.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hua XH, Newport J (1998). "Identification of a preinitiation step in DNA replication that is independent of origin recognition complex and cdc6, but dependent on cdk2". J. Cell Biol. 140 (2): 271–81. PMC 2132576. PMID 9442103.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rowles A, Tada S, Blow JJ (1999). "Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins". J. Cell. Sci. 112 ( Pt 12): 2011–8. PMC 3605702. PMID 10341218.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c d e Aparicio OM, Weinstein DM, Bell SP (1997). "Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase". Cell. 91 (1): 59–69. PMID 9335335.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Dutta A, Bell SP (1997). "Initiation of DNA replication in eukaryotic cells". Annu. Rev. Cell Dev. Biol. 13: 293–332. doi:10.1146/annurev.cellbio.13.1.293. PMID 9442876.
- ^ a b Labib K, Tercero JA, Diffley JF (2000). "Uninterrupted MCM2-7 function required for DNA replication fork progression". Science. 288 (5471): 1643–7. PMID 10834843.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Adachi Y, Usukura J, Yanagida M (1997). "A globular complex formation by Nda1 and the other five members of the MCM protein family in fission yeast". Genes Cells. 2 (7): 467–79. PMID 9366552.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Schwacha A, Bell SP (2001). "Interactions between two catalytically distinct MCM subgroups are essential for coordinated ATP hydrolysis and DNA replication". Mol. Cell. 8 (5): 1093–104. PMID 11741544.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Boyer PD (1993). "The binding change mechanism for ATP synthase--some probabilities and possibilities". Biochim. Biophys. Acta. 1140 (3): 215–50. PMID 8417777.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hingorani MM, Washington MT, Moore KC, Patel SS (1997). "The dTTPase mechanism of T7 DNA helicase resembles the binding change mechanism of the F1-ATPase". Proc. Natl. Acad. Sci. U.S.A. 94 (10): 5012–7. PMC 24622. PMID 9144181.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Labib K, Diffley JF, Kearsey SE (1999). "G1-phase and B-type cyclins exclude the DNA-replication factor Mcm4 from the nucleus". Nat. Cell Biol. 1 (7): 415–22. doi:10.1038/15649. PMID 10559985.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lei M, Tye BK (2001). "Initiating DNA synthesis: from recruiting to activating the MCM complex". J. Cell. Sci. 114 (Pt 8): 1447–54. PMID 11282021.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Leatherwood J (1998). "Emerging mechanisms of eukaryotic DNA replication initiation". Curr. Opin. Cell Biol. 10 (6): 742–8. PMID 9914182.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Mimura S, Takisawa H (1998). "Xenopus Cdc45-dependent loading of DNA polymerase alpha onto chromatin under the control of S-phase Cdk". EMBO J. 17 (19): 5699–707. doi:10.1093/emboj/17.19.5699. PMC 1170898. PMID 9755170.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Zou L, Stillman B (1998). "Formation of a preinitiation complex by S-phase cyclin CDK-dependent loading of Cdc45p onto chromatin". Science. 280 (5363): 593–6. PMID 9554851.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b c Mimura S, Masuda T, Matsui T, Takisawa H (2000). "Central role for cdc45 in establishing an initiation complex of DNA replication in Xenopus egg extracts". Genes Cells. 5 (6): 439–52. PMID 10886370.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tercero JA, Labib K, Diffley JF (2000). "DNA synthesis at individual replication forks requires the essential initiation factor Cdc45p". EMBO J. 19 (9): 2082–93. doi:10.1093/emboj/19.9.2082. PMC 305696. PMID 10790374.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aparicio OM, Stout AM, Bell SP (1999). "Differential assembly of Cdc45p and DNA polymerases at early and late origins of DNA replication". Proc. Natl. Acad. Sci. U.S.A. 96 (16): 9130–5. PMC 17744. PMID 10430907.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Zou L, Stillman B (2000). "Assembly of a complex containing Cdc45p, replication protein A, and Mcm2p at replication origins controlled by S-phase cyclin-dependent kinases and Cdc7p-Dbf4p kinase". Mol. Cell. Biol. 20 (9): 3086–96. PMC 85601. PMID 10757793.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Walter J, Newport J (2000). "Initiation of eukaryotic DNA replication: origin unwinding and sequential chromatin association of Cdc45, RPA, and DNA polymerase alpha". Mol. Cell. 5 (4): 617–27. PMID 10882098.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Masai H, Sato N, Takeda T, Arai K (1999). "CDC7 kinase complex as a molecular switch for DNA replication". Front. Biosci. 4: D834–40. PMID 10577390.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Jiang W, Wells NJ, Hunter T (1999). "Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6". Proc. Natl. Acad. Sci. U.S.A. 96 (11): 6193–8. PMC 26858. PMID 10339564.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Jiang W, McDonald D, Hope TJ, Hunter T (1999). "Mammalian Cdc7-Dbf4 protein [[kinase]] complex is essential for initiation of [[DNA replication]]". EMBO J. 18 (20): 5703–13. doi:10.1093/emboj/18.20.5703. PMC 1171637. PMID 10523313.
{{cite journal}}: URL–wikilink conflict (help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Kumagai H, Sato N, Yamada M; et al. (1999). "A novel growth- and cell cycle-regulated protein, ASK, activates human Cdc7-related kinase and is essential for G1/S transition in mammalian cells". Mol. Cell. Biol. 19 (7): 5083–95. PMC 84351. PMID 10373557.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hardy CF, Dryga O, Seematter S, Pahl PM, Sclafani RA (1997). "mcm5/cdc46-bob1 bypasses the requirement for the S phase activator Cdc7p". Proc. Natl. Acad. Sci. U.S.A. 94 (7): 3151–5. PMC 20337. PMID 9096361.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Blow JJ, Dutta A (2005). "Preventing re-replication of chromosomal DNA". Nat. Rev. Mol. Cell Biol. 6 (6): 476–86. doi:10.1038/nrm1663. PMC 2688777. PMID 15928711.
{{cite journal}}: Unknown parameter|month=ignored (help) Cite error: The named reference "pmid15928711" was defined multiple times with different content (see the help page). - ^ Takahashi TS, Wigley DB, Walter JC (2005). "Pumps, paradoxes and ploughshares: mechanism of the MCM2-7 DNA helicase". Trends Biochem. Sci. 30 (8): 437–44. doi:10.1016/j.tibs.2005.06.007. PMID 16002295.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b Pacek M, Walter JC (2004). "A requirement for MCM7 and Cdc45 in chromosome unwinding during eukaryotic DNA replication". EMBO J. 23 (18): 3667–76. doi:10.1038/sj.emboj.7600369. PMC 517609. PMID 15329670.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kubota Y, Takase Y, Komori Y; et al. (2003). "A novel ring-like complex of Xenopus proteins essential for the initiation of DNA replication". Genes Dev. 17 (9): 1141–52. doi:10.1101/gad.1070003. PMC 196047. PMID 12730133.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Masuda T, Mimura S, Takisawa H (2003). "CDK- and Cdc45-dependent priming of the MCM complex on chromatin during S-phase in Xenopus egg extracts: possible activation of MCM helicase by association with Cdc45". Genes Cells. 8 (2): 145–61. PMID 12581157.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Watson J, Baker T, Bell S, Gann A, Levine M, Losick R, Molecular Biology of the Gene 6th Edition, Pearson Education Inc, 2008. ISBN 080539592X
- ^ Elizabeth R. Barry; Stephen D. Bell (12/2006). "DNA Replication in the Archaea". Microbiology and Molecular Biology Reviews. 70 (4): 876–87. doi:10.1128/MMBR.00029-06. PMC 1698513. PMID 17158702.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ J. M. Berg; J. L. Tymoczko; L. Stryer "Biochemie", Springer, Heidelberg/Berlin 2003
- ^ a b Scott D McCulloch; Thomas A Kunkel (01/2008). "The fidelity of DNA synthesis by eukaryotic replicative and translesion synthesis polymerases". Cell Research. 18 (1): 148–61. doi:10.1038/cr.2008.4. PMID 18166979.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: multiple names: authors list (link) - ^ Pursell, Z.F.; et al. (2007). "Yeast DNA Polymerase ε Participates in Leading-Strand DNA Replication". Science. 317 (5834): 127–30. doi:10.1126/science.1144067. PMC 2233713. PMID 17615360.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ McGarry TJ, Kirschner MW (1998). "Geminin, an inhibitor of DNA replication, is degraded during mitosis". Cell. 93 (6): 1043–53. PMID 9635433.
{{cite journal}}: Unknown parameter|month=ignored (help)
