ABCA1: Difference between revisions
→Clinical significance: removing red links that are unlikely to have articles written about them |
RotogenRay (talk | contribs) →Physiological role: atherogenesis not angiogenesis& curcumin is not a polyphenol |
||
| Line 15: | Line 15: | ||
ABCA1 mediates the efflux of [[cholesterol]] and [[phospholipid]]s to lipid-poor [[apolipoprotein]]s (apo-A1 and apoE), which then form nascent [[high-density lipoprotein]]s (HDL). It also mediates the transport of lipids between Golgi and [[cell membrane]]. Since this protein is needed throughout the body it is [[Biosynthesis|expressed]] ubiquitously as a 220-[[Dalton (unit)|kDa]] protein. It is present in higher quantities in tissues that shuttle or are involved in the turnover of lipids such as the liver, the small intestine and adipose tissue.<ref>E. M. Wagner, F. Basso, C. S. Kim, M. J. A. Amar, "ABC lipid transporters", in AccessScience@McGraw-Hill</ref> |
ABCA1 mediates the efflux of [[cholesterol]] and [[phospholipid]]s to lipid-poor [[apolipoprotein]]s (apo-A1 and apoE), which then form nascent [[high-density lipoprotein]]s (HDL). It also mediates the transport of lipids between Golgi and [[cell membrane]]. Since this protein is needed throughout the body it is [[Biosynthesis|expressed]] ubiquitously as a 220-[[Dalton (unit)|kDa]] protein. It is present in higher quantities in tissues that shuttle or are involved in the turnover of lipids such as the liver, the small intestine and adipose tissue.<ref>E. M. Wagner, F. Basso, C. S. Kim, M. J. A. Amar, "ABC lipid transporters", in AccessScience@McGraw-Hill</ref> |
||
Factors that act upon the ABCA1 transporter's expression or its [[posttranslational modification]] are also molecules that are involved in its subsequent function like [[fatty acid]]s, cholesterol and also [[cytokine]]s and [[cyclic adenosine monophosphate]].<ref name="pmid16505586">{{cite journal | author = Yokoyama S | title = ABCA1 and biogenesis of HDL | journal = J. Atheroscler. Thromb. | volume = 13 | issue = 1 | pages = 1–15 |date=February 2006 | pmid = 16505586 | doi = 10.5551/jat.13.1| url = http://www.jstage.jst.go.jp/article/jat/13/1/13_1/_article }}</ref> |
Factors that act upon the ABCA1 transporter's expression or its [[posttranslational modification]] are also molecules that are involved in its subsequent function like [[fatty acid]]s, cholesterol and also [[cytokine]]s and [[cyclic adenosine monophosphate|cAMP]].<ref name="pmid16505586">{{cite journal | author = Yokoyama S | title = ABCA1 and biogenesis of HDL | journal = J. Atheroscler. Thromb. | volume = 13 | issue = 1 | pages = 1–15 |date=February 2006 | pmid = 16505586 | doi = 10.5551/jat.13.1| url = http://www.jstage.jst.go.jp/article/jat/13/1/13_1/_article }}</ref> |
||
Interactions between members of the apoliprotein family and ABCA1 activate multiple signalling pathways, including the [[JAK-STAT signaling pathway|JAK-STAT]], [[Protein Kinase A|PKA]], and [[Protein Kinase C|PKC]] pathways <ref name="pmid23847008 ">{{cite journal | author = Luu W, Sharpe LJ, Gelissen IC, Brown AJ | title = The role of signalling in cellular cholesterol homeostasis | journal = IUBMB Life | volume = 65 | issue = 8 | pages = 675–684 |date=August 2013 | pmid = 23847008 | doi = 10.1002/iub.1182| url = http://onlinelibrary.wiley.com/doi/10.1002/iub.1182/abstract;jsessionid=6413E9E73AC4A13EC015AFCE2D5D0F80.d01t01}}</ref> |
Interactions between members of the apoliprotein family and ABCA1 activate multiple signalling pathways, including the [[JAK-STAT signaling pathway|JAK-STAT]], [[Protein Kinase A|PKA]], and [[Protein Kinase C|PKC]] pathways <ref name="pmid23847008 ">{{cite journal | author = Luu W, Sharpe LJ, Gelissen IC, Brown AJ | title = The role of signalling in cellular cholesterol homeostasis | journal = IUBMB Life | volume = 65 | issue = 8 | pages = 675–684 |date=August 2013 | pmid = 23847008 | doi = 10.1002/iub.1182| url = http://onlinelibrary.wiley.com/doi/10.1002/iub.1182/abstract;jsessionid=6413E9E73AC4A13EC015AFCE2D5D0F80.d01t01}}</ref> |
||
Overexpression of ABCA1 has been reported to induce resistance to the anti-inflammatory |
Overexpression of ABCA1 has been reported to induce resistance to the anti-inflammatory [[Diarylheptanoid|diarylheptanoid]] [[antioxidant]] [[Curcumin]].<ref>{{cite journal | author = Bachmeier BE, Iancu CM, Killian PH, Kronski E, Mirisola V, Angelini G, Jochum M, Nerlich AG,Pfeffer U.| year = 2009 | title = Overexpression of the ATP binding cassette gene ABCA1 determines resistance to Curcumin in M14 melanoma cells | journal = Mol Cancer| volume = 8 | pages = 129–141 | pmid = 20030852 | doi=10.1186/1476-4598-8-129 | pmc=2804606}}</ref> |
||
Downregulation of ABCA1 in senescent macrophages disrupts the cell's ability to remove cholesterol from its cytosoplasm, leading the cells to promote the pathologic |
Downregulation of ABCA1 in senescent macrophages disrupts the cell's ability to remove cholesterol from its cytosoplasm, leading the cells to promote the pathologic [[atherogenesis]] (blood vessel thickening/hardening) which "plays a central role in common age-associated diseases such as atherosclerosis, cancer, and macular degeneration" <ref>{{cite journal | author = Sene A, Khan AA,, et al| year = 2013 | title = Impaired Cholesterol Efflux in Senescent Macrophages Promotes Age-Related Macular Degeneration | journal = Cell Metabolism| volume = 17 | pages = 549–561 | doi=10.1016/j.cmet.2013.03.009}}</ref> Knockout mouse models of [[Age-related macular degeneration|AMD]] treated with agonists that increase ABCA1 in loss of function and gain of function experiments demonstrated the protective role of elevating ABCA1 in regulating [[angiogenesis]] in eye disease. Human data from patients and controls were used to demonstrate the translation of mouse findings in human disease.<ref>http://www.faqs.org/patents/app/20130317090</ref> |
||
== Clinical significance == |
== Clinical significance == |
||
Revision as of 19:18, 25 July 2014
Template:PBB ATP-binding cassette transporter ABCA1 (member 1 of human transporter sub-family ABCA), also known as the cholesterol efflux regulatory protein (CERP) is a protein which in humans is encoded by the ABCA1 gene.[1] This transporter is a major regulator of cellular cholesterol and phospholipid homeostasis.
Tangier Disease
It was discovered that a mutation in the ABCA1 protein is responsible for causing Tangier's Disease by several groups in 1998. Gerd Schmitz's group in Germany[2] and Michael Hayden's group in British Columbia[3] were using standard genetics techniques and DNA from family pedigrees to locate the mutation. Richard Lawn's group at CV Therapeutics in Palo Alto, CA used cDNA microarrays, which were relatively new at the time, to assess gene expression profiles from cell lines created from normal and effected individuals.[4] They showed cell lines from patients with Tangier's disease showed differential regulation of the ABCA1 gene. Subsequent sequencing of the gene identified the mutations. This group received an award from the American Heart Association for their discovery.[5] Tangier disease has been identified in nearly 100 patients worldwide, and patients have a broad range of biochemical and clinical phenotypes as over 100 different mutations have been identified in ABCA1 resulting in the disease.[6]
Function
The membrane-associated protein encoded by this gene is a member of the superfamily of ATP-binding cassette (ABC) transporters. ABC proteins transport various molecules across extra- and intracellular membranes. ABC genes are divided into seven distinct subfamilies (ABCA, MDR/TAP, MRP, ALD, OABP, GCN20, White). This protein is a member of the ABCA subfamily. Members of the ABCA subfamily comprise the only major ABC subfamily found exclusively in multicellular eukaryotes. With cholesterol as its substrate, this protein functions as a cholesterol efflux pump in the cellular lipid removal pathway.[7][8]
While the complete 3D-structure of ABCA1 remains relatively unknown, there has been some determination of the c-terminus. The ABCA1 c-terminus contains a PDZ domain, responsible for mediating protein-protein interactions, as well as a VFVNFA motif essential for lipid efflux activity.[6]
Physiological role
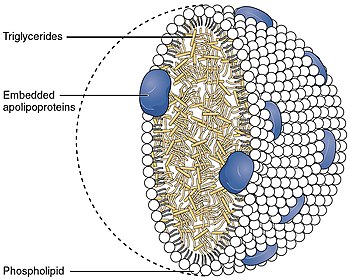
ABCA1 mediates the efflux of cholesterol and phospholipids to lipid-poor apolipoproteins (apo-A1 and apoE), which then form nascent high-density lipoproteins (HDL). It also mediates the transport of lipids between Golgi and cell membrane. Since this protein is needed throughout the body it is expressed ubiquitously as a 220-kDa protein. It is present in higher quantities in tissues that shuttle or are involved in the turnover of lipids such as the liver, the small intestine and adipose tissue.[9]
Factors that act upon the ABCA1 transporter's expression or its posttranslational modification are also molecules that are involved in its subsequent function like fatty acids, cholesterol and also cytokines and cAMP.[10]
Interactions between members of the apoliprotein family and ABCA1 activate multiple signalling pathways, including the JAK-STAT, PKA, and PKC pathways [11]
Overexpression of ABCA1 has been reported to induce resistance to the anti-inflammatory diarylheptanoid antioxidant Curcumin.[12] Downregulation of ABCA1 in senescent macrophages disrupts the cell's ability to remove cholesterol from its cytosoplasm, leading the cells to promote the pathologic atherogenesis (blood vessel thickening/hardening) which "plays a central role in common age-associated diseases such as atherosclerosis, cancer, and macular degeneration" [13] Knockout mouse models of AMD treated with agonists that increase ABCA1 in loss of function and gain of function experiments demonstrated the protective role of elevating ABCA1 in regulating angiogenesis in eye disease. Human data from patients and controls were used to demonstrate the translation of mouse findings in human disease.[14]
Clinical significance
Mutations in this gene have been associated with Tangier disease and familial high-density lipoprotein deficiency. ABCA1 has been shown to be reduced in Tangier disease which features physiological deficiencies of HDL.[15][16] Leukocytes ABCA1 gene expression is upregulated in postmenopausal women receiving hormone replacement therapy (HRP).[17]
Interactive pathway map
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "Statin_Pathway_WP430".
See also
Interactions
ABCA1 has been shown to interact with:
References
- ^ Luciani MF, Denizot F, Savary S, Mattei MG, Chimini G (May 1994). "Cloning of two novel ABC transporters mapping on human chromosome 9". Genomics. 21 (1): 150–9. doi:10.1006/geno.1994.1237. PMID 8088782.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bodzioch M, Orsó E, Klucken J, Langmann T, Böttcher A, Diederich W, Drobnik W, Barlage S, Büchler C, Porsch-Ozcürümez M, Kaminski WE, Hahmann HW, Oette K, Rothe G, Aslanidis C, Lackner KJ, Schmitz G (August 1999). "The gene encoding ATP-binding cassette transporter 1 is mutated in Tangier disease". Nature Genetics. 22 (4): 347–51. doi:10.1038/11914. PMID 10431237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brooks-Wilson A, Marcil M, Clee SM, Zhang LH, Roomp K, van Dam M, Yu L, Brewer C, Collins JA, Molhuizen HO, Loubser O, Ouelette BF, Fichter K, Ashbourne-Excoffon KJ, Sensen CW, Scherer S, Mott S, Denis M, Martindale D, Frohlich J, Morgan K, Koop B, Pimstone S, Kastelein JJ, Genest J, Hayden MR (August 1999). "Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency". Nature Genetics. 22 (4): 336–45. doi:10.1038/11905. PMID 10431236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Lawn RM, Wade DP, Garvin MR, Wang X, Schwartz K, Porter JG, Seilhamer JJ, Vaughan AM, Oram JF (October 1999). "The Tangier disease gene product ABC1 controls the cellular apolipoprotein-mediated lipid removal pathway". The Journal of Clinical Investigation. 104 (8): R25–31. doi:10.1172/JCI8119. PMC 481052. PMID 10525055.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "American Heart Association Selects CV Therapeutics' Discovery of Role Of 'Good' Cholesterol-Regulating Gene as Top Ten 1999 Research Advances In Heart Disease". PR Newswire Association. 2000-01-03. Retrieved 2009-05-08.
- ^ a b Brunham LR, Singaraja RR, Hayden MR (August 2006). "Variations of a gene: rare and common variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis". Annual Review of Nutrition. 26: 105–129. doi:10.1146/annurev.nutr.26.061505.111214. PMID 16704350.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Entrez Gene: ABCA1 ATP-binding cassette, sub-family A (ABC1), member 1".
- ^ Schmitz G, Langmann T (April 2001). "Structure, function and regulation of the ABC1 gene product". Curr. Opin. Lipidol. 12 (2): 129–40. doi:10.1097/00041433-200104000-00006. PMID 11264984.
- ^ E. M. Wagner, F. Basso, C. S. Kim, M. J. A. Amar, "ABC lipid transporters", in AccessScience@McGraw-Hill
- ^ Yokoyama S (February 2006). "ABCA1 and biogenesis of HDL". J. Atheroscler. Thromb. 13 (1): 1–15. doi:10.5551/jat.13.1. PMID 16505586.
- ^ Luu W, Sharpe LJ, Gelissen IC, Brown AJ (August 2013). "The role of signalling in cellular cholesterol homeostasis". IUBMB Life. 65 (8): 675–684. doi:10.1002/iub.1182. PMID 23847008.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bachmeier BE, Iancu CM, Killian PH, Kronski E, Mirisola V, Angelini G, Jochum M, Nerlich AG,Pfeffer U. (2009). "Overexpression of the ATP binding cassette gene ABCA1 determines resistance to Curcumin in M14 melanoma cells". Mol Cancer. 8: 129–141. doi:10.1186/1476-4598-8-129. PMC 2804606. PMID 20030852.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Sene A, Khan AA,; et al. (2013). "Impaired Cholesterol Efflux in Senescent Macrophages Promotes Age-Related Macular Degeneration". Cell Metabolism. 17: 549–561. doi:10.1016/j.cmet.2013.03.009.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ http://www.faqs.org/patents/app/20130317090
- ^ Ordovas JM (March 2000). "ABC1: the gene for Tangier disease and beyond". Nutr. Rev. 58 (3 Pt 1): 76–9. doi:10.1111/j.1753-4887.2000.tb01843.x. PMID 10812922.
- ^ Oram JF, Vaughan AM (June 2000). "ABCA1-mediated transport of cellular cholesterol and phospholipids to HDL apolipoproteins". Curr. Opin. Lipidol. 11 (3): 253–60. doi:10.1097/00041433-200006000-00005. PMID 10882340.
- ^ Darabi M, Rabbani M, Ani M, Zarean E, Panjehpour M, Movahedian A (2011). "Increased leukocyte ABCA1 gene expression in post-menopausal women on hormone replacement therapy". Gynecol. Endocrinol. 27 (9): 701–5. doi:10.3109/09513590.2010.507826. PMID 20807164.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fitzgerald ML, Morris AL, Rhee JS, Andersson LP, Mendez AJ, Freeman MW (September 2002). "Naturally occurring mutations in the largest extracellular loops of ABCA1 can disrupt its direct interaction with apolipoprotein A-I". J. Biol. Chem. 277 (36): 33178–87. doi:10.1074/jbc.M204996200. PMID 12084722.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Buechler C, Bared SM, Aslanidis C, Ritter M, Drobnik W, Schmitz G (November 2002). "Molecular and functional interaction of the ATP-binding cassette transporter A1 with Fas-associated death domain protein". J. Biol. Chem. 277 (44): 41307–10. doi:10.1074/jbc.C200436200. PMID 12235128.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Buechler C, Boettcher A, Bared SM, Probst MC, Schmitz G (May 2002). "The carboxyterminus of the ATP-binding cassette transporter A1 interacts with a beta2-syntrophin/utrophin complex". Biochem. Biophys. Res. Commun. 293 (2): 759–65. doi:10.1016/S0006-291X(02)00303-0. PMID 12054535.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shimizu Y, Iwai S, Hanaoka F, Sugasawa K (January 2003). "Xeroderma pigmentosum group C protein interacts physically and functionally with thymine DNA glycosylase". EMBO J. 22 (1): 164–73. doi:10.1093/emboj/cdg016. PMC 140069. PMID 12505994.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Further reading
- Tam SP, Mok L, Chimini G, Vasa M, Deeley RG (2006). "ABCA1 mediates high-affinity uptake of 25-hydroxycholesterol by membrane vesicles and rapid efflux of oxysterol by intact cells". Am J Physiol Cell Physiol. 291 (3): C490–502. doi:10.1152/ajpcell.00055.2006. PMID 16611739.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Oram JF (2003). "ATP-binding cassette transporter A1 and cholesterol trafficking". Curr. Opin. Lipidol. 13 (4): 373–81. doi:10.1097/00041433-200208000-00004. PMID 12151852.
- Hong SH, Rhyne J, Zeller K, Miller M (2003). "ABCA1(Alabama): a novel variant associated with HDL deficiency and premature coronary artery disease". Atherosclerosis. 164 (2): 245–50. doi:10.1016/S0021-9150(02)00106-5. PMID 12204794.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Kozak M (2003). "Emerging links between initiation of translation and human diseases". Mamm. Genome. 13 (8): 401–10. doi:10.1007/s00335-002-4002-5. PMID 12226704.
- Joyce C, Freeman L, Brewer HB, Santamarina-Fojo S (2004). "Study of ABCA1 function in transgenic mice". Arterioscler. Thromb. Vasc. Biol. 23 (6): 965–71. doi:10.1161/01.ATV.0000055194.85073.FF. PMID 12615681.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Singaraja RR, Brunham LR, Visscher H; et al. (2004). "Efflux and atherosclerosis: the clinical and biochemical impact of variations in the ABCA1 gene". Arterioscler. Thromb. Vasc. Biol. 23 (8): 1322–32. doi:10.1161/01.ATV.0000078520.89539.77. PMID 12763760.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - Nofer JR, Remaley AT (2005). "Tangier disease: still more questions than answers". Cell. Mol. Life Sci. 62 (19–20): 2150–60. doi:10.1007/s00018-005-5125-0. PMID 16235041.
- Yokoyama S (2006). "ABCA1 and biogenesis of HDL". J. Atheroscler. Thromb. 13 (1): 1–15. doi:10.5551/jat.13.1. PMID 16505586.
- Schmitz G, Schambeck CM (2006). "Molecular defects in the ABCA1 pathway affect platelet function". Pathophysiol. Haemost. Thromb. 35 (1–2): 166–74. doi:10.1159/000093563. PMID 16855366.
External links
- ABCA1 human gene location in the UCSC Genome Browser.
- ABCA1 human gene details in the UCSC Genome Browser.