Garenoxacin
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
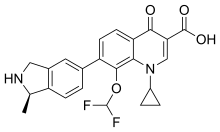
| Formula | C23H20F2N2O4 |
| Molar mass | 426.420 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Garenoxacin (INN) is a quinolone antibiotic for the treatment of Gram-positive and Gram-negative bacterial infections.
Garenoxacin was discovered by Toyama Chemical Co., Ltd. of Tokyo, Japan, and is currently being marketed in Japan under the tradename Geninax. Schering-Plough holds worldwide rights for garenoxacin, except for Japan, South Korea, and China.
On February 13, 2006, Schering-Plough announced that the United States Food and Drug Administration had accepted the New Drug Application (NDA) for garenoxacin, and had been granted a 10-month review.[1] As of 2015, however, it has not been approved in the US.
Schering-Plough later withdrew its application to the United States Food and Drug Administration, FDA, (August 20, 2006) for approval of the antibiotic Garenoxacin.[2]
The European Medicines Agency (EMA) had also been formally notified by Schering-Plough Europe (July 25, 2007) of its decision to withdraw the application for a centralized marketing authorization for garenoxacin as well.[3][4][5] Based on the CHMP review of the data regarding safety and efficacy (risk/benefit), the CHMP considered the application for garenoxacin to be unapprovable.[6]
References
- ^ "Drugs.com, Schering-Plough Reports Garenoxacin NDA Accepted for FDA Review". Retrieved 2008-03-25.
- ^ "Schering-Plough pulls its garenoxacin app".
- ^ http://www.medicalnewstoday.com/articles/78052.php
- ^ "Garenoxacin mesylate: Withdrawn application". European Medicines Agency (EMA). Retrieved 13 July 2020.
- ^ "Schering-Plough Europe withdraws its marketing authorisation applicationfor Garenoxacin mesylate". European Medicines Agency (EMA) (Press release). Retrieved 13 July 2020.
- ^ http://www.emea.europa.eu/humandocs/PDFs/EPAR/garenoxacinmesylate/H-747-WAR.pdf[permanent dead link]
