Lignan
The lignans are a large group of polyphenols found in plants.[1] Some examples of lignans are enterolignans, enterodiol and enterolactone.[1]
Etymology
From lign- (Latin, "wood") + -an (chemical suffix).
Technical definition
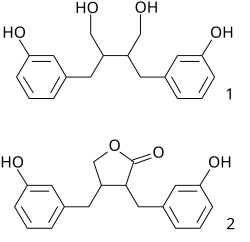
Plant lignans are polyphenolic substances derived from phenylalanine via dimerization of substituted cinnamic alcohols (see cinnamic acid), known as monolignols, to a dibenzylbutane skeleton 2. This reaction is catalysed by oxidative enzymes and is often controlled by dirigent proteins.
Structure

Many natural products, known as phenylpropanoids, are built up of C6C3 units (n-propylbenzene skeleton 1) derived from cinnamyl units just as terpene chemistry builds on isoprene units. Structure 3 is a neolignan, a structure formed by joining the two propylbenzene residues at other than the β-carbon atom of the propyl side chain.
As diet
When a part of the human diet, some plant lignans are metabolized by intestinal bacteria to mammalian lignans enterodiol (1) and enterolactone (2).[2][3][4] Lignans that can be metabolized to mammalian lignans are pinoresinol, lariciresinol, secoisolariciresinol, matairesinol, hydroxymatairesinol, syringaresinol and sesamin. Lignans are one of the major classes of phytoestrogens, which are estrogen-like chemicals and also act as antioxidants. The other classes of phytoestrogens are isoflavones and coumestans.
Food sources
Flax seed and sesame seed contain higher levels of lignans than most other foods.[citation needed] The principal lignan precursor found in flaxseed is secoisolariciresinol diglucoside.[citation needed] Other sources of lignans include cereals (rye, wheat, oat and barley - rye being the richest source), soybeans, cruciferous vegetables such as broccoli and cabbage, and some fruit, particularly apricots and strawberries.[1]
Secoisolariciresinol and matairesinol were the first plant lignans identified in foods. Typically, lariciresinol and pinoresinol contribute about 75% to the total lignan intake whereas secoisolariciresinol and matairesinol contribute only about 25%.[1] Lignans are some of the secondary metabolites present in Cannabis sativa.[5]
Sources of lignans:[6]
| Source | Amount per 100 g |
|---|---|
| Flaxseed | 300,000 μg (0.3 g) |
| Sesame seed | 29,000 μg (29 mg) |
| Brassica vegetables | 185 - 2321 μg |
| Grains | 7 - 764 μg |
| Red wine | 91 μg |
A 2007 study[7] shows the complexity of mammalian lignan precursors in the diet. In the table below are a few examples of the 22 analyzed species and the 24 lignans identified in this study.
Mammalian lignan precursors as aglycones (μg/100 g). Major compound(s) in bold.
| Foodstuff | Pinoresinol | Syringaresinol | Sesamin | Lariciresinol | Secoisolariciresinol | Matairesinol | Hydroxymatairesinol |
|---|---|---|---|---|---|---|---|
| Flaxseed | 871 | 48 | not detected | 1780 | 165759 | 529 | 35 |
| Sesame seed | 47136 | 205 | 62724 | 13060 | 240 | 1137 | 7209 |
| Rye bran | 1547 | 3540 | not detected | 1503 | 462 | 729 | 1017 |
| Wheat bran | 138 | 882 | not detected | 672 | 868 | 410 | 2787 |
| Oat bran | 567 | 297 | not detected | 766 | 90 | 440 | 712 |
| Barley bran | 71 | 140 | not detected | 133 | 42 | 42 | 541 |
Pharmacology
Lignans are under basic research for their potential anti-inflammatory or antioxidant activity in laboratory models of human diseases.[8] Hinokinin is an emerging lignan with many pharmacologically useful properties.[9]
See also
References
- ^ a b c d "Lignans". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 2010. Retrieved 31 July 2017.
- ^ Heinonen, S; Nurmi, T; Liukkonen, K; Poutanen, K; Wähälä, K; Deyama, T; Nishibe, S; Adlercreutz, H (2001). "In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol". Journal of Agricultural and Food Chemistry. 49 (7): 3178–86. doi:10.1021/jf010038a. PMID 11453749.
- ^ Axelson, M; Sjövall, J; Gustafsson, B. E.; Setchell, K. D. (1982). "Origin of lignans in mammals and identification of a precursor from plants". Nature. 298 (5875): 659–60. Bibcode:1982Natur.298..659A. doi:10.1038/298659a0. PMID 6285206.
- ^ Borriello, S. P.; Setchell, K. D.; Axelson, M; Lawson, A. M. (1985). "Production and metabolism of lignans by the human faecal flora". The Journal of Applied Bacteriology. 58 (1): 37–43. doi:10.1111/j.1365-2672.1985.tb01427.x. PMID 2984153.
- ^ Flores-Sanchez, Isvett Josefina; Verpoorte, Robert (2008-10-01). "Secondary metabolism in cannabis". Phytochemistry Reviews. 7 (3): 615–639. doi:10.1007/s11101-008-9094-4. ISSN 1568-7767.
- ^ Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (2005). "Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol". Br. J. Nutr. 93 (3): 393–402. doi:10.1079/BJN20051371. PMID 15877880.
- ^ Smeds AI; Eklund, Patrik C.; Sjöholm, Rainer E.; Willför, Stefan M.; Nishibe, Sansei; Deyama, Takeshi; Holmbom, Bjarne R.; et al. (2007). "Quantification of a Broad Spectrum of Lignans in Cereals, Oilseeds, and Nuts". J. Agric. Food Chem. 55 (4): 1337–1346. doi:10.1021/jf0629134. PMID 17261017.
- ^ Korkina, L; Kostyuk, V; De Luca, C; Pastore, S (2011). "Plant phenylpropanoids as emerging anti-inflammatory agents". Mini Reviews in Medicinal Chemistry. 11 (10): 823–35. doi:10.2174/138955711796575489. PMID 21762105.
- ^ https://www.researchgate.net/profile/Maria_Carla_Marcotullio/publication/265791867_Hinokinin_an_Emerging_Bioactive_Lignan/links/5448f4da0cf22b3c14e33f3c/Hinokinin-an-Emerging-Bioactive-Lignan.pdf?origin=publication_detail.
{{cite web}}: Missing or empty|title=(help)CS1 maint: url-status (link)
