Casopitant
Appearance
 | |
| Clinical data | |
|---|---|
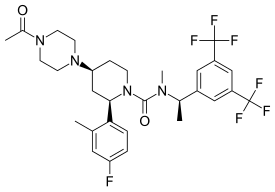
| Other names | (2R,4S)-4-(4-Acetylpiperazin-1-yl)-N-{(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethyl}-2-(4-fluoro-2-methylphenyl)-N-methylpiperidine-1-carboxamide |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C30H35F7N4O2 |
| Molar mass | 616.26 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Casopitant (INN,[1]: 208 tentative trade names Rezonic (U.S.), Zunrisa (Europe)) is an neurokinin 1 receptor (NK1R) antagonist undergoing research for the treatment of chemotherapy-induced nausea and vomiting (CINV).[2] It is currently under development by GlaxoSmithKline (GSK).
In July 2008, the company filed a marketing authorisation application with the European Medicines Agency. The application was withdrawn in September 2009 because GSK decided that further safety assessment was necessary.[3]
See also
References
- ^ "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List56" (PDF). World Health Organization. 2006. Retrieved 17 November 2016.
- ^ Lohr L (2008). "Chemotherapy-induced nausea and vomiting". Cancer J. 14 (2): 85–93. doi:10.1097/PPO.0b013e31816a0f07. PMID 18391612.
- ^ "GlaxoSmithKline withdraws its marketing authorisation application for Zunrisa" (PDF). London: EMEA. 13 October 2009. Retrieved 21 December 2009.[permanent dead link]
