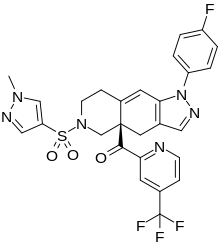
Relacorilant
Appearance
 | |
| Clinical data | |
|---|---|
| Other names | CORT-125134 |
| Routes of administration | By mouth |
| Drug class | Antiglucocorticoid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C27H22F4N6O3S |
| Molar mass | 586.566 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Relacorilant (developmental code name CORT-125134) is an antiglucocorticoid which is under development by Corcept Therapeutics for the treatment of Cushing's syndrome.[1] It is also under development for the treatment of solid tumors and alcoholism.[1][2] The drug is a nonsteroidal compound and acts as an antagonist of the glucocorticoid receptor.[1] As of December 2017, it is in phase II clinical trials for Cushing's syndrome and phase I/II clinical studies for solid tumors, while the clinical phase for alcoholism is unknown.[1]
See also
References
- ^ a b c d http://adisinsight.springer.com/drugs/800041622
- ^ Veneris JT, Darcy KM, Mhawech-Fauceglia P, Tian C, Lengyel E, Lastra RR, Pejovic T, Conzen SD, Fleming GF (2017). "High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer". Gynecol. Oncol. 146 (1): 153–160. doi:10.1016/j.ygyno.2017.04.012. PMID 28456378.
External links
