Sulfotransferase
Appearance
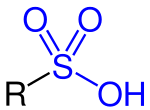
Sulfotransferases EC 2.8.2.- are transferase enzymes that catalyze the transfer of a sulfo group from a donor molecule to an acceptor alcohol or amine.[1] The most common sulfo group donor is 3'-phosphoadenosine-5'-phosphosulfate (PAPS). In the case of alcohol as acceptor, the product is a sulfate (R-OSO3−), whereas an amine leads to a sulfamate (R-NH-SO3−). Both reactive groups for a sulfonation via sulfotransferases may be part of a protein, lipid, carbohydrate or steroid.[2]
Examples
The following are examples of sulfotransferases:
- carbohydrate sulfotransferase: CHST1, CHST2, CHST3, CHST4, CHST5, CHST6, CHST7, CHST8, CHST9, CHST10, CHST11, CHST12, CHST13, CHST14
- galactose-3-O-sulfotransferase: GAL3ST1, GAL3ST2, GAL3ST3, GAL3ST4
- heparan sulfate 2-O-sulfotransferase: HS2ST1,
- heparan sulfate 3-O-sulfotransferase: HS3ST1, HS3ST2, HS3ST3A1, HS3ST3A2, HS3ST3B1, HS3ST3B2, HS3ST4, HS3ST5, HS3ST6
- heparan sulfate 6-O-sulfotransferase: HS6ST1, HS6ST2, HS6ST3
- N-deacetylase/N-sulfotransferase: NDST1, NDST2, NDST3, NDST4
- tyrosylprotein sulfotransferase: TPST1, TPST2
- uronyl-2-sulfotransferase
- Estrone sulfotransferase
- Chondroitin 4-sulfotransferase
- other: SULT1A1, SULT1A2, SULT1A3, SULT1A4, SULT1B1, SULT1C2, SULT1C3, SULT1C4, SULT1D1P, SULT1E1, SULT2A1, SULT2B1, SULT4A1, SULT6B1
See also
- List of EC numbers (EC 2)#EC 2.8.2: Sulfotransferases
- Wikipedia:MeSH D08#MeSH D08.811.913.817 --- sulfur group transferases .28EC 2.8.29
References
- ^ Negishi M; Pedersen LG; Petrotchenko E; et al. (2001). "Structure and function of sulfotransferases". Arch. Biochem. Biophys. 390 (2): 149–57. doi:10.1006/abbi.2001.2368. PMID 11396917.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Rath VL, Verdugo D, Hemmerich S (2004). "Sulfotransferase structural biology and inhibitor discovery". Drug Discov. Today. 9 (23): 1003–11. doi:10.1016/S1359-6446(04)03273-8. PMID 15574316.
External links
- Sulfotransferases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
