Ytterbium(III) chloride
This article's lead section may be too short to adequately summarize the key points. (September 2013) |

| |

| |
| Names | |
|---|---|
| IUPAC name
Ytterbium(III) chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.715 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| YbCl3 | |
| Molar mass | 279.40 g/mol |
| Appearance | White powder |
| Density | 4.06 g/cm3 (solid) |
| Melting point | 854 °C (1,569 °F; 1,127 K)[1] |
| Boiling point | 1,453 °C (2,647 °F; 1,726 K)[1] |
| 17 g/100 mL (25 °C) | |
| Structure | |
| Monoclinic, mS16 | |
| C12/m1, No. 12 | |
| Related compounds | |
Other anions
|
Ytterbium(III) oxide |
Other cations
|
Terbium(III) chloride, Lutetium(III) chloride |
| Supplementary data page | |
| Ytterbium(III) chloride (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Ytterbium(III) chloride (YbCl3) is an inorganic chemical compound. It reacts with NiCl2 to form a very effective catalyst for the reductive dehalogenation of aryl halides.[2] It is poisonous if injected, and mildly toxic by ingestion. It is an experimental teratogen, known to irritate the skin and eyes. When heated to decomposition it emits toxic fumes of Cl−.[3]
History
Ytterbium, a lanthanide series element, was discovered in 1878 by the Swiss chemist Jean-Charles Galissard de Marignac, who named the element after a town (Ytterby) in Sweden.[4] The first synthesis of YbCl3 in the literature was that of Jan Hoogschagen in 1946.[5] YbCl3 is now a commercially available source of Yb3+ ions and therefore of significant chemical interest.
Chemical properties
The valence electron configuration of Yb+3 (from YbCl3) is 4f135s25p6, which has crucial implications for the chemical behaviour of Yb+3. Also, the size of Yb+3 governs its catalytic behaviour and biological applications. For example, while both Ce+3 and Yb+3 have a single unpaired f electron, Ce+3 is much larger than Yb+3 because lanthanides become much smaller with increasing effective nuclear charge as a consequence of the f electrons not being as well shielded as d electrons.[4] This behavior is known as the lanthanide contraction. The small size of Yb+3 produces fast catalytic behavior and an atomic radius (0.99 Å) comparable to many biologically important ions.[4]
The gas-phase thermodynamic properties of this chemical are difficult to determine because the chemical can disproportionate to form [YbCl6]−3 or dimerize.[6] The Yb2Cl6 species was detected by electron impact (EI) mass spectrometry as (Yb2Cl5+).[6] Additional complications in obtaining experimental data arise from the myriad of low-lying f-d and f-f electronic transitions.[7] Despite these issues, the thermodynamic properties of YbCl3 have been obtained and the C3V symmetry group has been assigned based upon the four active infrared vibrations.[7]
Preparation
YbCl3 is prepared from Yb2O3 with either high-temperature carbon tetrachloride gas,[8] or hot hydrochloric acid followed by drying at high temperature.[9]
- 2 Yb2O3 + 3 CCl4(g) → 4 YbCl3(s) + 3 CO2(g)
- Yb2O3 + 6 HCl(g) → 2 YbCl3(s) + 3 H2O
In practice[clarification needed] there are better ways to prepare YbCl3 for lab use. The aqueous HCl/ammonium chloride route[clarification needed] is unsophisticated[clarification needed] but very effective. Alternatively hydrated YbCl3 may be dehydrated using a variety of reagents, particularly trimethylsilyl chloride. Other methods have been published including reacting the finely powdered metal with mercuric chloride at high temperature in a sealed tube.[citation needed] A variety of routes to solvated YbCl3 have been reported[citation needed] including reaction of the metal with various halocarbons in the present of a donor solvent such as THF, or dehydration of the hydrated chloride using trimethylsilyl or thionyl chloride, again in a solvent such as THF.
Uses
Catalysis
YbCl3, with a single unpaired electron, acts as a Lewis acid in order to fill the 4f orbital. The Lewis acidic nature of YbCl3 allows YbCl3 to coordinate (usually as [YbCl2]+) in transition states to catalyze alkylation reactions, such as the aldol reaction[10] and the Pictet-Spengler reaction.[11]
- Aldol reaction
The aldol reaction is a versatile reaction in synthetic organic chemistry. YbCl3 serves a Lewis acid catalyst which aids the Pd(0) catalyzed decarboxylative aldol reaction between a ketone enolate and an aldehyde. Transition states A and B show the coordination method of the ytterbium salt as a Lewis acid.[10] For the depicted decarboxylative Aldol reaction with R = tert-butyl and R’ = -(CH2)2Ph, the reaction yields show YbCl3 is an effective Lewis acid catalyst:
| Metal salt[10] | % yield of 2 |
|---|---|
| FeCl3 | 40 |
| ZnCl2 | 68 |
| CuCl2 | 40 |
| LaCl3 | 60 |
| YbCl3 | 93 |
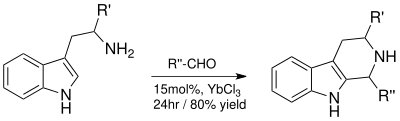
- Pictet-Spengler reaction
The Pictet-Spengler reaction produces a valuable tetrahydro-β-carboline ring system which can be later used for synthetically prepared indole alkaloids.[11] The Lewis acid catalyzed reaction with YbCl3 gave excellent yields and reduced reaction times from four days to 24 hours.[11]
- Esterification
The small size of Yb3+ provides fast catalysis, but at the cost of selectivity. For example, the mono-acetylation ofmeso-1,2-diols is fastest (2 h) with YbCl3, but chemoselectivity for the mono-acetylated product is low (50%) compared to CeCl3 (23 h, 85%).[12]
- Acetalisation
Ytterbium(III) chloride is a powerful catalyst for the formation of acetals using trimethyl orthoformate.[13] In comparison with cerium(III) chloride and erbium(III) chloride, the ytterbium salt was found to be the most effective. Excellent yields are obtained from a variety of aldehydes within a few minutes at room temperature, as in the example above involving an acid-sensitive aldehyde.
Biology
YbCl3 is an NMR shift reagent that produces different resonances with nuclei in contact with the YbCl3 versus those nuclei not in contact with the shift reagent.[14] Generally paramagnetic species, such as a (lanthanide)+3 ion, are desirable shift reagents.[14] Membrane biology has been greatly influenced by YbCl3, where39K+ and23Na+ ion movement is critical in establishing electrochemical gradients.[14] Nerve signaling is a fundamental aspect of life that may be probed with YbCl3 using NMR techniques. YbCl3 may also be used as a calcium ion probe, in a fashion similar to a sodium ion probe.[15]
YbCl3 is also used to track digestion in animals. Certain additives to swine feed, such as probiotics, may be added to either solid feed or drinking liquids. YbCl3 travels with the solid food and therefore helps determine which food phase is ideal to incorporate the food additive.[16] The YbCl3 concentration is quantified by inductively coupled plasma mass spectrometry to within 0.0009 μg/mL.[4] YbCl3 concentration versus time yields the flow rate of solid particulates in the animal's digestion. The animal is not harmed by the YbCl3 since YbCl3 is simply excreted in fecal matter and no change in body weight, organ weight, or hematocrit levels has been observed in mice.[15]
The catalytic nature of YbCl3 also has an application in DNA microarrays, or so called DNA “chips”.[17] YbCl3 led to a 50–80 fold increase in fluorescein incorporation into target DNA, which could revolutionize infectious disease detection (such as a rapid test for tuberculosis).[17]
References
- ^ a b Walter Benenson; John W. Harris; Horst Stöcker (2002). Handbook of Physics. Springer. p. 781. ISBN 0-387-95269-1.
- ^ Zhang, Y. et al. Synth. Commun. 27, 4327, (1997)
- ^ "Ytterbiumchloride; Ytterbium trichloride; Ytterbium(III) chloride Ytterbium chloride(YbCl3) dictionary - Guidechem.com". www.guidechem.com. Retrieved 2016-11-30.
- ^ a b c d Evans, C.H. Biochemistry of the Lanthanides; Plenum: New York, 1990.
- ^ Hoogschagen, J. (1946). "The light absorption in the near infra red region of praseodymium, samarium and ytterbium solutions". Physica. 11 (6): 513–517. Bibcode:1946Phy....11..513H. doi:10.1016/S0031-8914(46)80020-X.
- ^ a b Chervonnyi, A.D.; Chervonnaya, N.A. (2004). "Thermodynamic Properties of Ytterbium Chlorides". Russ. J. Inorg. Chem. (Engl. Transl.). 49 (12): 1889–1897.
- ^ a b Zasorin, E.Z. (1988). Russ. J. Phys. Chem. (Engl. Transl.). 62 (4): 441–447.
{{cite journal}}: Missing or empty|title=(help) - ^ Goryushkin, V.F.; Zalymova, S.A.; Poshevneva, A.I. (1990). Russ. J. Inorg. Chem. (Engl. Transl.). 35 (12): 1749–1752.
{{cite journal}}: Missing or empty|title=(help) - ^ Jörg, S.; Seifert, H.J. (1998). "Ternary chlorides in the systems ACl/YbCl3 (A=Cs,Rb,K)". Thermochimica Acta. 318 (1–2): 29–37. doi:10.1016/S0040-6031(98)00326-8.
- ^ a b c Lou, S.; Westbrook, J.A.; Schaus, S.E. (2004). "Decarboxylative aldol reactions of allyl beta-keto esters via heterobimetallic catalysis". Journal of the American Chemical Society. 126 (37): 11440–11441. doi:10.1021/ja045981k. PMID 15366881.
- ^ a b c Srinivasan, N.; Ganesan, A. (2003). "Highly efficient Lewis acid-catalysed Pictet–Spengler reactions discovered by parallel screening". Chemical Communications (Cambridge, England) (7): 916–917. doi:10.1039/b212063a. PMID 12739676.
- ^ Clarke, P.A. (2002). "Selective mono-acylation of meso- and C2-symmetric 1,3- and 1,4-diols". Tetrahedron Letters. 43 (27): 4761–4763. doi:10.1016/S0040-4039(02)00935-8.
- ^ Luche, Jean-Louis; Gemal, André Luis (1978). "Efficient synthesis of acetals catalysed by rare earth chlorides". Journal of the Chemical Society, Chemical Communications. 1978 (22): 976–977. doi:10.1039/c39780000976.
- ^ a b c Hayer, M.K.; Riddell, F.G. (1984). "Shift reagents for 39K Nmr". Inorganica Chimica Acta. 92 (4): L37–L39. doi:10.1016/S0020-1693(00)80044-4.
- ^ a b Shinohara, A.; Chiba, M.; Inaba, Y. (2006). "Comparative study of the behavior of terbium, samarium, and ytterbium intravenously administered in mice". Journal of Alloys and Compounds. 408–412: 405–408. doi:10.1016/j.jallcom.2004.12.152.
- ^ Ohashi, Y.; Umesaki, Y.; Ushida, K. (2004). "Transition of the probiotic bacteria, Lactobacillus casei strain Shirota, in the gastrointestinal tract of a pig". International Journal of Food Microbiology. 96 (1): 61–66. doi:10.1016/j.ijfoodmicro.2004.04.001. PMID 15358506.
- ^ a b Browne, K.A. (2002). "Metal ion-catalyzed nucleic acid alkylation and fragmentation". Journal of the American Chemical Society. 124 (27): 7950–7962. doi:10.1021/ja017746x. PMID 12095339.