Pancreatic cancer: Difference between revisions
→Exocrine: ref |
→Endocrine: ref |
||
| Line 36: | Line 36: | ||
===Endocrine=== |
===Endocrine=== |
||
There are a number of types, all of which can be considered rare. They form part of a wider classification of [[neuroendocrine tumor]]s ("NETs"), with which they have much in common, and those forming in the pancreas are referred to as "PanNETs" for convenience. They are divided into "functioning" and "non-functioning" types. The functioning types produce endocrine [[hormone]]s such as [[insulin]], [[gastrin]], and [[glucagon]], often in large quantities that give rise to serious symptoms such as [[low blood sugar]], which makes them likely to be detected relatively early. The non-functioning types, which represent some 90% of PanNETs, produce no hormones, and typically no symptoms at an early stage. They are often only diagnosed after the cancer has spread to other parts of the body. Surgical procedures are similar to those for exocrine tumors. Chemotherapy has shown little effectiveness against PanNETs, but for functioning tumors a class of drugs called [[Hormonal_therapy_(oncology)#Somatostatin_analogs|somatostatin analogs]] are effective in reducing the excessive production of hormones and the associated problems.<ref>{{cite journal|last1=Öberg|first1=K|last2=Knigge|first2=U|last3=Kwekkeboom|first3=D|last4=Perren|first4=A|last5=ESMO Guidelines Working|first5=Group|title=Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.|journal=Annals of oncology : official journal of the European Society for Medical Oncology / ESMO|date=2012 Oct|volume=23 Suppl 7|pages=vii124-30|pmid=22997445|accessdate=30 September 2014}}</ref> The range of possible outcomes vary greatly; some types have a very high survival rate after surgery while others have a poor outlook. As all this group are rare, treatment should be undertaken in a specialized centre with experience of them. |
There are a number of types, all of which can be considered rare. They form part of a wider classification of [[neuroendocrine tumor]]s ("NETs"), with which they have much in common, and those forming in the pancreas are referred to as "PanNETs" for convenience.<ref>{{cite journal|last1=Burns|first1=WR|last2=Edil|first2=BH|title=Neuroendocrine pancreatic tumors: guidelines for management and update.|journal=Current treatment options in oncology|date=2012 Mar|volume=13|issue=1|pages=24-34|pmid=22198808|accessdate=30 September 2014}}</ref> They are divided into "functioning" and "non-functioning" types. The functioning types produce endocrine [[hormone]]s such as [[insulin]], [[gastrin]], and [[glucagon]], often in large quantities that give rise to serious symptoms such as [[low blood sugar]], which makes them likely to be detected relatively early. The non-functioning types, which represent some 90% of PanNETs, produce no hormones, and typically no symptoms at an early stage. They are often only diagnosed after the cancer has spread to other parts of the body. Surgical procedures are similar to those for exocrine tumors. Chemotherapy has shown little effectiveness against PanNETs, but for functioning tumors a class of drugs called [[Hormonal_therapy_(oncology)#Somatostatin_analogs|somatostatin analogs]] are effective in reducing the excessive production of hormones and the associated problems.<ref>{{cite journal|last1=Öberg|first1=K|last2=Knigge|first2=U|last3=Kwekkeboom|first3=D|last4=Perren|first4=A|last5=ESMO Guidelines Working|first5=Group|title=Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up.|journal=Annals of oncology : official journal of the European Society for Medical Oncology / ESMO|date=2012 Oct|volume=23 Suppl 7|pages=vii124-30|pmid=22997445|accessdate=30 September 2014}}</ref> The range of possible outcomes vary greatly; some types have a very high survival rate after surgery while others have a poor outlook. As all this group are rare, treatment should be undertaken in a specialized centre with experience of them. |
||
<ref>{{cite journal|last1=Burns|first1=WR|last2=Edil|first2=BH|title=Neuroendocrine pancreatic tumors: guidelines for management and update.|journal=Current treatment options in oncology|date=2012 Mar|volume=13|issue=1|pages=24-34|pmid=22198808|accessdate=30 September 2014}}</ref> |
|||
==Signs and symptoms== |
==Signs and symptoms== |
||
Revision as of 17:16, 30 September 2014
| Pancreatic cancer | |
|---|---|
| Specialty | Oncology, gastroenterology |
Pancreatic cancer occurs when cancer cells develop from the pancreas, a glandular organ located behind the stomach. Signs and symptoms of pancreatic cancer may include abdominal or back pain, yellow skin, unexplained weight loss, light colored stools, dark urine and loss of appetite. Early on there are usually no symptoms.[1] Symptoms that are specific enough to suspect pancreatic cancer often do not appear until the disease is already in an advanced stage.[1] By the time of diagnosis the cancer has usually spread to other parts of the body.[2]
Pancreatic cancer is rare in those younger than 40, and the median age of diagnosis is 71.[3] Risk factors include: smoking, obesity, diabetes, and certain rare genetic conditions including: multiple endocrine neoplasia type 1 and hereditary nonpolyposis colon cancer among others.[1] About 25% of cases are attributable to tobacco smoking,[4] while 5-10% of cases are linked to inherited genes.[3] Infiltrating ductal adenocarcinoma is the most common type of pancreatic cancer, making up over 80% of cases,[5] and references to pancreatic cancer often refer only to that type. It arises within the part of the pancreas that makes digestive enzymes, known as the exocrine pancreas. One to two percent arise from islet cells, and are classified as neuroendocrine tumors. There are also a number of other types of pancreatic cancer.[2] Diagnosis is usually based on a combination of imaging tests such as ultrasound and computed tomography, blood tests such as CEA and CA 19-9 and biopsy. This allows the disease to be divided into five stages.[1]
The most important form of prevention is to cease smoking, after which the risk of the disease returns to normal within 20 years.[2] Other recommendations include limiting alcohol intake and eating a healthy diet.[6] Screening the general population has not been found to be effective.[6] For people affected by the disease treatments may include: surgery, radiation therapy, chemotherapy, or a combination of treatments. Recommendations are partly based on the cancer stage. Surgery may be done in an effort to cure the disease or to try to improve quality of life without trying to cure. Pain management and medications to improve digestion are sometimes needed.[1] Early palliative care is recommended even in those who are receiving active treatment.[7][8]
In 2012 pancreatic cancers of all types caused 330,000 deaths globally, the seventh most common cause of deaths due to cancer.[2] In the United States it is the fourth most common cause of deaths due to cancer.[9] The disease occurs more often in the developed world, which had 68% of new cases in 2012.[2] It often has poor outcomes with the average percentage alive for at least one and five years being 25% and 5% respectively.[2][10] In localized disease where the cancer is small (< 2 cm) the number alive at five years is approximately 20%.[11] For those with neuroendocrine cancer the number alive at five years is better at 65%.[2] In the United States, as of 2006, the economic costs of pancreatic cancer are estimated at $8.6 billion.[12]
Classification
All the cancers that can affect the pancreas can first be divided into the 99% that occur in the exocrine (or "non-endocrine") parts of the pancreas. There are several types of these, but their diagnosis and treatment have much in common. The remaining 1% of pancreatic cancers are in the endocrine parts of the pancreas, and often have different symptoms and treatment to the exocrine types. Both groups mainly (but not exclusively) occur in people over 40, and are slightly more common in men, but some rare sub-types mainly occur in women or children.[13] For both the only curative treatment is surgery, and for most sub-types the outcomes are typically poor.
Exocrine
The exocrine group is dominated by pancreatic adenocarcinoma ("invasive" and "ductal" may be added to this term), which is by far the most common type, representing about 85% of all pancreatic cancers,[3] and covered in detail in other sections. The next most common, acinar cell carcinoma of the pancreas represents 5% of exocrine pancreas cancers. Like "functioning" endocrine cancers, it may cause over-production of pancreatic products, in this case digestive enzymes, which may produce symptoms including skin rashes and joint pain. Cystadenocarcinoma represents 1% and has a better prognosis than other types.[14] Pancreatoblastoma is a rare form, mostly occurring in childhood, and with a relatively good prognosis. Other exocrine cancers include adenosquamous carcinomas, signet ring cell carcinomas, hepatoid carcinomas, colloid carcinomas, undifferentiated carcinomas, and undifferentiated carcinomas with osteoclast-like giant cells.[15]
Pancreatic mucinous cystic neoplasms are a broad group of pancreas tumors that have varying malignant potential. They are being detected at a greatly increased rate as CT scans become more powerful and common, and discussion continues as how best to assess and treat them, as many are benign.[16]
Endocrine
There are a number of types, all of which can be considered rare. They form part of a wider classification of neuroendocrine tumors ("NETs"), with which they have much in common, and those forming in the pancreas are referred to as "PanNETs" for convenience.[17] They are divided into "functioning" and "non-functioning" types. The functioning types produce endocrine hormones such as insulin, gastrin, and glucagon, often in large quantities that give rise to serious symptoms such as low blood sugar, which makes them likely to be detected relatively early. The non-functioning types, which represent some 90% of PanNETs, produce no hormones, and typically no symptoms at an early stage. They are often only diagnosed after the cancer has spread to other parts of the body. Surgical procedures are similar to those for exocrine tumors. Chemotherapy has shown little effectiveness against PanNETs, but for functioning tumors a class of drugs called somatostatin analogs are effective in reducing the excessive production of hormones and the associated problems.[18] The range of possible outcomes vary greatly; some types have a very high survival rate after surgery while others have a poor outlook. As all this group are rare, treatment should be undertaken in a specialized centre with experience of them. [19]
Signs and symptoms


Early pancreatic cancer usually does not cause symptoms, so that the disease is typically not diagnosed until it has spread beyond the pancreas itself.[20] This is one of the key factors in the poor survival rate.
Common symptoms before diagnosis include:
- Pain in the upper abdomen or back, often spreading from around the stomach to the back. The location of the pain can indicate the part of the pancreas where a tumor is located. The pain may be worst at night and may increase over time to become "severe and unremitting". It may be slightly relieved by bending forwards.[21] In the UK, about half of new cases of pancreatic cancer are diagnosed following a visit to a hospital emergency department for pain or jaundice, or both. Up to 2/3 of patients have abdominal pain, 46% accompanied by jaundice, with 13% having jaundice without pain.[22]
- Painless jaundice (yellow tint to whites of eyes (sclera) or yellowish skin, possibly in combination with darkened urine)[23] when a cancer of the head of the pancreas (75% of cases)[24] obstructs the common bile duct as it runs through the pancreas. This may also cause pale-colored stool and steatorrhea. The jaundice may be associated with itching as the salt from excess bile can cause skin irritation.
- Unexplained weight loss (cachexia)
Other symptoms
- Indigestion (dyspepsia) or heartburn.[22]
- Poor appetite or nausea and vomiting
- Diarrhea, loose stools.
- Trousseau's syndrome, in which blood clots form spontaneously in the portal blood vessels, the deep veins of the extremities, or the superficial veins anywhere on the body, may be associated with pancreatic cancer, and is found in about 10% of cases.[25]
- Pulmonary embolisms due to pancreatic cancers producing blood clotting chemicals.
- Diabetes mellitus, or elevated blood sugar levels. Many patients with pancreatic cancer develop diabetes months to even years before they are diagnosed with pancreatic cancer, suggesting new onset diabetes in an elderly individual may be an early warning sign of pancreatic cancer.[2][26]
- Clinical depression has been reported in association with pancreatic cancer in some 10-20% of cases, and can be a hindrance to optimal management. The depression may be there before the cancer is diagnosed, and is perhaps caused by the cancer. But the mechanism for this association is not known.[22][27]
- Symptoms of pancreatic cancer metastasis. Typically, pancreatic cancer first metastasizes to regional lymph nodes, and later to the liver or to the peritoneal cavity, large intestine or lungs;[28][29] it rarely metastasizes to bone or brain.[30]
Risk factors
Risk factors for pancreatic cancer include:[2] [22][23][31][32]
- Age, gender and race. The risk of developing pancreatic cancer increases with age. Most cases occur after age 65,[2] while cases before age 40 are uncommon. The disease is somewhat more common in men than women, and in the United States is over 1.5 times more common in African Americans, though incidence in Africa is low.[2]
- Cigarette smoking is the best established avoidable risk factor for pancreatic cancer, approximately doubling risk among long-term smokers, with the risk increasing with the number of cigarettes smoked and the years of smoking. The risk declines slowly after smoking cessation, taking some 20 years to return to that of non-smokers.[33]
- Obesity; a BMI greater than 35 is associated with a risk ratio of 1.55.[22]
- Family history: 5–10% of pancreatic cancer cases have an inherited component,[3] with many patients having a family history of pancreatic cancer. Most of the genes involved have not been identified.[3] Hereditary pancreatitis gives a greatly increased lifetime risk of pancreatic cancer, which has been variously calculated as 35–54% to the age of 75.[34][35][36] Screening for early pancreatic cancer may be offered to individuals with hereditary pancreatitis on a research basis.[37] Some patients may choose to have their pancreas surgically removed to prevent cancer developing in the future.[38]
- Pancreatic cancer has been associated with the following syndromes: autosomal recessive ataxia-telangiectasia and autosomal dominantly inherited mutations in the BRCA2 gene and PALB2 gene, Peutz-Jeghers syndrome due to mutations in the STK11 tumor suppressor gene, hereditary non-polyposis colon cancer (Lynch syndrome), familial adenomatous polyposis, and the familial atypical multiple mole melanoma-pancreatic cancer syndrome (FAMMM-PC) due to mutations in the CDKN2A tumor suppressor gene.[39][40] There may also be a history of familial pancreatitis.[39]
- Chronic pancreatitis has been linked, but is not known to be causal. The risk of pancreatic cancer in individuals with familial pancreatitis is particularly high.
- Diabetes mellitus is both a risk factor for pancreatic cancer, and, as noted earlier, new onset diabetes can be an early sign of the disease.
- Helicobacter pylori infection[41][42]
- Gingivitis or periodontal disease[43]
- Diet (other than alcohol) is not generally accepted as a risk factor, although some individual studies have found dietary factors such as diets low in vegetables and fruits,[44] high in red meat or processed meat,[45] sugar-sweetened drinks (soft drinks),[46] In particular, limited epidemiological studies link the common soft drink sweetener fructose with growth of pancreatic cancer cells.[47] or coffee.
- Partial gastrectomy[48][49]
Alcohol
While the association between alcohol abuse and pancreatitis is well established, considerable research has failed to firmly establish alcohol consumption as a risk factor for pancreatic cancer. Overall, the association is consistently weak and the majority of studies have found no association.[50][51] Although drinking alcohol excessively is a major cause of chronic pancreatitis, which in turn predisposes to pancreatic cancer, chronic pancreatitis associated with alcohol consumption is less frequently a precursor for pancreatic cancer than other types of chronic pancreatitis.[52]
Diagnosis
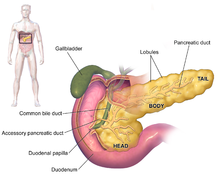

Most patients with pancreatic cancer experience pain, weight loss, or jaundice.[53]
Pain is present in 80% to 85% of patients with locally advanced or advanced metastatic disease. The pain is usually felt in the upper abdomen as a dull ache that radiates straight through to the back. It may be intermittent and made worse by eating. Weight loss can be profound; it can be associated with anorexia, early satiety, diarrhoea, or steatorrhea. Jaundice is often accompanied by pruritus and dark urine. Painful jaundice is present in approximately one-half of patients with locally unresectable disease, while painless jaundice is present in approximately one-half of patients with a potentially resectable and curable lesion.
The initial presentation varies according to the location of the cancer on the pancreas, which anatomists divide (going from left to right on most diagrams) into the thick head, the neck, and the tapering body, ending in the tail. About 60-70% of adenocarcinomas are in the head, and 20-25% in the body or tail.[3] Malignancies in the pancreatic body or tail usually present with pain and weight loss, while those in the head of the gland typically present with steatorrhea, weight loss, and jaundice.
The recent onset of atypical diabetes mellitus, a history of recent but unexplained thrombophlebitis (Trousseau sign), or a previous attack of pancreatitis are sometimes noted. Courvoisier's sign defines the presence of jaundice and a painlessly distended gallbladder as strongly indicative of pancreatic cancer, and may be used to distinguish pancreatic cancer from gallstones. Tiredness, irritability and difficulty eating because of pain also exist. Pancreatic cancer is often discovered during the course of the evaluation of aforementioned symptoms.
Liver function tests can show a combination of results indicative of bile duct obstruction (raised conjugated bilirubin, γ-glutamyl transpeptidase and alkaline phosphatase levels). CA19-9 (carbohydrate antigen 19.9) is a tumor marker that is frequently elevated in pancreatic cancer. However, it lacks sensitivity and specificity. When a cutoff above 37 U/mL is used, this marker has a sensitivity of 77% and specificity of 87% in discerning benign from malignant disease. CA 19-9 might be normal early in the course, and could be elevated because of benign causes of biliary obstruction.[54] Imaging studies, such as computed tomography (CT scan) and endoscopic ultrasound (EUS) can be used to identify the location and form of the cancer. The definitive diagnosis is made by an endoscopic needle biopsy or surgical excision of the radiologically suspicious tissue. Endoscopic ultrasound is often used to visually guide the needle biopsy procedure.[55] Nonetheless, pancreatic cancer is usually staged using a CT scan. In fact, a histologic diagnosis is not usually required for resection of the tumor, rather histologic analysis helps determine which chemotherapeutic regimen to start.[56]
Staging
The cancer staging system used internationally for pancreatic cancer is that of the American Joint Committee on Cancer and Union for International Cancer Control, so AJCC-UICC, which makes an important distinction within Stage II, between tumors that are classed as "borderline resectable" because they do not involve the celiac axis or superior mesenteric artery, and "unresectable". Surgery is likely to be possible for the former, but is not for the latter. These are T3 and T4 respectively in the associated TNM staging system.[57] A simpler practical classification groups cases as "resectable", "borderline resectable", and "unresectable" because locally advanced or metastatic.[58]
-
Stage T1 pancreatic cancer
-
Stage T2 pancreatic cancer
-
Stage T3 pancreatic cancer
-
Stage T4 pancreatic cancer
-
Pancreatic cancer in the lymph nodes
-
Pancreatic cancer metastasized
Mechanism

The development of pancreatic cancer may involve the over-expression of oncogenes, inactivation of tumor suppressor genes or the deregulation of various signaling proteins.[59] Mutations leading to carcinoma may be accelerated by genetic or environmental factors and other risk factors already described. Specific mutations vary among and even within the cyto-histologic categories discussed below.
Exocrine pancreas cancers

The most common form of pancreatic cancer (ductal adenocarcinoma) is typically characterized by moderately to poorly differentiated glandular structures on microscopic examination. There is typically considerable formation of fibrous tissue (desmoplasia) around the tumour. This creates an environment that is short of blood vessels (hypovascular) and so of oxygen (Tumor hypoxia).[3] It is thought that this prevents many chemotherapy drugs from reaching the tumor, as one factor making the cancer especially hard to treat.[60] Pancreatic cancer has an immunohistochemical profile that is similar to hepatobiliary cancers (e.g. cholangiocarcinoma) and some stomach cancers; thus, it may not always be possible to be certain that a tumour found in the pancreas arose from it.
The genetic events that cause ductal adenocarcinoma have been well characterized. The most common are KRAS mutations (96%), CDKN2A mutations/deletions (75%), TP53 mutations (55%), SMAD4 deletions/mutations (50%), and SWI/SNF mutations/deletions (35%).[61][62]

Pancreatic carcinoma is thought to arise from progressive tissue changes. Three types of precancerous lesion are recognized: pancreatic intraepithelial neoplasia – a microscopic lesions of the pancreas, intraductal papillary mucinous neoplasms and mucinous cystic neoplasms both of which are macroscopic lesions.[63] The cellular origin of these lesions is debated.
Pancreatic neuroendocrine tumors
Endocrine pancreatic tumors have been variously called islet cell tumors, pancreas endocrine tumors (PETs), and pancreatic neuroendocrine tumors (PNETs).[64] The annual clinically recognized incidence is low, about five per one million person-years.[15] However, autopsy studies incidentally identify PETs in up to 1.5%[65] most of which would remain inert and asymptomatic.[65]
The majority of PNETs are usually categorized as benign[66][67][68] but the definition of malignancy in pancreas endocrine tumors has been ambiguous. A small subset of endocrine pancreatic tumors are incontrovertible pancreatic endocrine cancers, that make up about 1% of pancreas cancers.[15][64] Low- to intermediate-grade neuroendocrine carcinomas of the pancreas may be called islet cell tumors. Some sources have also termed these pancreatic carcinoid,[64] a practice that has sometimes been strongly condemned.[citation needed] Definitional migration has caused some complexity of PNET classification,[64] which has adversely affected what is known about the epidemiology and natural history of these tumors.[64] It is probable that some of these tumors have been included in ICD-O-3 histology classifications 8240–8245, in that they were labeled pancreatic carcinoid tumours[64][69] but most islet cell carcinomas have been coded as ICD-O-3 system 8150–8155.[64]
The more aggressive endocrine pancreatic cancers are known as pancreatic neuroendocrine carcinomas (PNEC). Similarly, there has likely been a degree of admixture of PNEC and extrapulmonary small cell carcinoma.[citation needed]
Prevention
Apart from not smoking, the American Cancer Society recommends keeping a healthy weight, and increasing consumption of fruits, vegetables, and whole grains, while decreasing red meat intake, although there is no consistent evidence this will prevent or reduce pancreatic cancer specifically.[70][71] In 2006, a large prospective cohort study of over 80,000 subjects failed to prove a definite association.[72] The evidence in support of this lies mostly in small case-control studies.[44]
A Harvard study from 2007 showed a modest inverse trend between blood circulation of B vitamins, such as B12, B6, and folate and pancreatic cancer incidence, but not when the vitamins were ingested in tablet form.[73] However, the results of a meta-analysis of randomized trials by Rothwell and colleagues indicate that taking a daily low-dose aspirin regimen for more than five years decreases the risk of developing pancreatic adenocarcinoma (ductal pancreatic cancer) by 75%.[74]
Screening
It is generally agreed that general screening of large groups is not at present likely to be effective, and outside clinical trials there are no programmes for this. The European Society for Medical Oncology recommends regular screening with endoscopic ultrasound and MRI/CT imaging for those at high risk from inherited genetics,[5] in line with other recommendations,[20][75] which may also include CT.[75]
Management
Exocrine cancer
The first and most crucial clinical decision to be made after diagnosis is whether surgical removal of the tumor is possible, as only this offers hope of a cure. This will require a tumor that has not metastasized, and will then depend on the location and spread of the tumor. In particular the tumor will be examined through CT to see how it relates to the major blood vessels passing close to the pancreas. An abutment of the tumor, defined as the tumor touching up to 180° of a blood vessel's circumference, may be operable, but encasement, defined as more 180° engaged, is not. The general health of the patient must also be assessed, though age in itself is not an obstacle to surgery.[76]
Chemotherapy and, to a lesser extent, radiotherapy, are likely to be offered to most patients, whether or not surgery is possible. Management of pancreatic cancer should be in the hands of a multidisciplinary team including specialists in several aspects of oncology, and is therefore best conducted in larger centers.[3][77]
Surgery

Treatment of pancreatic cancer depends on the stage of the cancer.[78] Although only localized cancer is considered suitable for surgery with curative intent at present, only 20% of cases present with localized disease at diagnosis.[79] The evidence as to resectability is hard to assess, and it often turns out in surgery that it is not possible to successfully remove the tumor without damaging other vital parts. To avoid such unnecessary surgery, a diagnostic laparoscopy can be performed to enable a much clearer idea of the outcome of a full operation.[80] Surgery can also be performed for palliation, if the malignancy is invading or compressing the duodenum or colon. In such cases, bypass surgery might overcome the obstruction and improve quality of life but is not intended as a cure.[55]
The Whipple procedure is the most common attempted curative surgical treatment for cancers involving the head of the pancreas. This procedure involves removing the pancreatic head and the curve of the duodenum together (pancreato-duodenectomy), making a bypass for food from stomach to jejunum (gastro-jejunostomy) and attaching a loop of jejunum to the cystic duct to drain bile (cholecysto-jejunostomy). It can be performed only if the patient is likely to survive major surgery and if the cancer is localized without invading local structures or metastasizing. It can, therefore, be performed in only the minority of cases. Cancers of the tail of the pancreas can be resected using a procedure known as a distal pancreatectomy, which often includes splenectomy or removal of the spleen.[3] This is today often done using keyhole or laparoscopic surgery.[3]
After surgery, adjuvant chemotherapy with gemcitabine or 5-FU should be offered if the patient is fit after surgery.[5] There has been controversy as to whether it is beneficial to add radiotherapy since the 1980s,[81] and the European Society for Medical Oncology recommend that this should only be used for patients in clinical trials.[5] However it is more likely to be used in the USA.[20]
Those with inoperable pancreatic cancer may have significant abdominal pain. A celiac plexus block (CPB), which destroys the nerves that transmit pain from the abdomen, is a safe and effective way to reduce the pain. CPB generally reduces the need to use pain killers like opioids, which have significant negative side effects.[82]
-
Diagram showing how the pancreas and bowel are joined back together after Whipple's operation
-
Diagram showing the area to be removed for pylorus-preserving pancreaticoduodenectomy
-
Diagram showing the pancreas joined to the small bowel after pylorus preserving surgery for pancreatic cancer
-
Diagram showing how the bowel is joined back together after a total pancreatectomy
Radiation
The role of radiation therapy after potentially curative surgery has been controversial for many years, with a continuing tendency for clinicians in the US to be more ready to use adjuvant radiation therapy than those in Europe. Many clinical trials since the 1980s, testing a variety of treatment regimes, have failed to settle the matter conclusively.[3][25][83]
Chemotherapy
In people not suitable for resection with curative intent, palliative chemotherapy may be used to improve quality of life and gain a modest survival benefit. Gemcitabine was approved by the United States Food and Drug Administration in 1997,[84] after a clinical trial reported improvements in quality of life and a 5-week improvement in median survival duration in patients with advanced pancreatic cancer. This marked the first FDA approval of a chemotherapy drug primarily for a nonsurvival clinical trial endpoint. Gemcitabine is administered intravenously on a weekly basis.
Chemotherapy using gemcitabine alone was the standard for the years following, as a number of trials testing it in varying dosage regimes and in combination with other drugs failed to demonstrate significantly better outcomes. However the combination of gemcitabine with Erlotinib was found to increase survival, and Erlotinib was licensed by the FDA for use in pancreatic cancer. The FOLFIRINOX chemotherapy regimen using four drugs was found more effective than gemcitabine, but with serious side effects, and thus only suitable for patients with good performance status. This is also true of protein-bound paclitaxel or nab-paclitaxel, which was licensed by the FDA in 2013 for this purpose. By the end of 2013, both FOLFIRINOX and nab-paclitaxel were regarded as good choices for those patients who were able to withstand the side-effects, with gemcitabine remaining an option for those who were not. A head to head trial between the two new options is awaited, and trials investigating other variations continue. However, the changes of the last few years have only increased average survival times by a few months.[84]
Neuroendocrine tumors
The majority of these tumors are histologically benign. Treatment of pancreatic endocrine tumors, including the less common malignant tumors, may include:
- Watchful waiting: incidentally identified small tumors, for example on a computed tomography (CT) scan performed for other purposes, may conceptually not always need intervention, but criteria for watchful waiting are unclear.
- Surgery: tumors within the pancreas only (localized tumors), or with limited metastases, may be removed. For localized tumors, the surgical procedure is much less extensive than the types of surgery used to treat pancreatic adenocarcinoma.
- Hormone therapy: if the tumor is not amenable to surgical removal and is causing symptoms by secreting functional hormones, a synthetic hormone analog medication, octreotide, may lessen the symptoms, and sometimes also slows tumor growth.
- Radiation therapy: occasionally used if there is pain due to anatomic extension, such as metastasis to bone.
- Radiolabeled hormone: some PNETs absorb a hormone called norepinephrine and these may respond to nuclear medicine medication, radiolabeled MIBG therapy (or, experamentally, other hormones), given intravenously.
- Chemotherapy and targeted medication for PNETs receive Wikipedia discussion in the relevant section of that article.
Palliative care
Palliative care is medical care which focuses on treatment of symptoms from serious illness, like cancer, and improving quality of life.[85] Because pancreatic adenocarcinoma, the most common type of pancreatic cancer, is one of the most aggressive cancers, it is usually diagnosed after it has progressed to an advanced stage, and there are fewer treatment options compared to other cancers, life expectancy of less than one year is expected in 80-90% of patients, who will benefit from palliative care as a treatment of symptoms.[86]
Palliative care will focus not on treating the underlying cancer, but on treating symptoms such as pain or nausea, and can assist in decision making such as when or if hospice care will be beneficial.[87] Pain can be managed with medications such as opioids or through procedural intervention such as celiac plexus blocks, which alters the nerves that may be causing pain. Other symptoms/complications that can be treated with palliative surgery are biliary or intestinal obstruction. Palliative care can also help treat depression that often comes with diagnosis of pancreatic cancer, as well as fatigue and cachexia.[88]
Prognosis
| Clinical stage | Five-year survival (%) - U.S., diagnoses 1992-98 | |
|---|---|---|
| Exocrine pancreatic cancer | neuroendocrine treated with surgery | |
| IA / I | 14 | 61 |
| IB | 12 | |
| IIA / II | 7 | 52 |
| IIB | 5 | |
| III | 3 | 41 |
| IV | 1 | 16 |
Pancreatic adenocarcinoma and the other less common exocrine cancers have a very poor prognosis, as they are resistant to treatment and usually cause no early symptoms and so are diagnosed late, when the cancer is already locally advanced or has spread to other parts of the body.[3] Outcomes with pancreatic endocrine tumors, many of which are benign and completely without clinical symptoms, are much better, and even those cases not able to be treated by surgery have a 5-year survival rate of 16%.[90]
For locally advanced and metastatic pancreatic adenocarcinoma, which collectively represent over 80%[11] to 85-90%[39] of cases, numerous recent trials comparing chemotherapy regimes have shown increased average survival rates, but not to above one year.[3][84] Together with lung cancer, pancreatic cancer as a whole has shown the least improvement in US survival rates of all common cancers over the three decades to 2013, although 5-year survival has improved from 2% in cases diagnosed in 1975-77, and 4% in 1987-89 diagnoses, to 6% in 2002-08.[11]
Epidemiology

Globally, as of 2012, pancreatic cancer resulted in 330,000 deaths,[2] up from 310,000 in 2010 and 200,000 in 1990.[92] In 2014, an estimated 46,000 people in the US are expected to be diagnosed with pancreatic cancer and 40,000 to die of it.[3] Pancreatic cancer has one of the highest fatality rates of all cancers, and is the fourth-highest cancer killer among both men and women worldwide.[93] Although it accounts for only 2.5% of new cases, pancreatic cancer is responsible for 6% of cancer deaths each year.[94]
The rates of incidence vary greatly, with those in the developed world generally being higher. The disease is slightly more common in men than women.[3][2] In the United States the risk for African Americans is over 50% greater than for whites, but the rates in Africa, like East Asia, are much lower than those in North America or Europe. The United States, Central and eastern Europe, and Argentina and Uruguay all have high rates of incidence.[2]
Research
As of 2014, worldwide efforts are under way to understand pancreatic cancer on many levels,[95][96] and there are several fundamental unanswered questions. Research on pancreatic cancer has been recognized[97] as an area in need of prioritization due to limited progress over recent decades.
The nature of the genetic changes that lead to the disease are being intensely scrutinized, for example by the Australian Pancreatic Genome Initiative[98] as part of the International Cancer Genome Consortium. These and others have uncovered[99] the key role played by genes such as KRAS and p53 in the disease’s development. A key question is the timing of key events in the disease’s progression – particularly how and when it spreads (metastasizes), and how these are affected by lifestyle risk factors such as obesity and smoking.
Research on early detection is ongoing, for example the European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC)[100] trial is aiming to determine whether regular screening is appropriate for people with a family history of the disease, or who have hereditary pancreatitis.
Parallel to this, efforts are underway to develop new drugs to target the disease, or to test existing drugs that are currently not used to treat it. Some of these involve treatments to target cancer cells themselves using targeted therapies.[101] Others aim to target the tissue surrounding the pancreatic tumour (the stroma or microenvironment).[102] The availability of new genetically engineered mouse models has substantially advanced this research in recent years.[103] A third key strand of research on treating the disease is immunotherapy – particularly using oncolytic viruses.[104]
Another key area of interest is in assessing whether keyhole surgery (laparoscopy) would be better than Whipple’s Procedure (pancreaticoduodenectomy) in treating the disease surgically,[105] particularly in terms of recovery time.
The limited success of outcomes after surgery has led to a number of trials that were running in 2014 to test outcomes using chemotherapy or radiochemotherapy before surgery. This had previously not been found to be helpful, but is being trialed again, using drug combinations which have emerged from the many trials of post-operative therapies, such as FOLFIRINOX.[3]
See also
- Gastrointestinal cancer
- Pancreatic Cancer Action (organization in the UK)
- Lustgarten Foundation for Pancreatic Cancer Research (organization in the US)
References
- ^ a b c d e "Pancreatic Cancer Treatment (PDQ®) Patient Version". National Cancer Institute. 17 April 2014. Retrieved 8 June 2014.
- ^ a b c d e f g h i j k l m n World Cancer Report 2014. World Health Organization. 2014. pp. Chapter 5.7. ISBN 9283204298.
- ^ a b c d e f g h i j k l m n o p Ryan DP, Hong TS, Bardeesy N (September 2014). "Pancreatic adenocarcinoma". N. Engl. J. Med. 371 (11): 1039–49. doi:10.1056/NEJMra1404198. PMID 25207767.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 319. doi:10.3322/caac.21190. PMID 23856911.
- ^ a b c d Seufferlein, T; Bachet, JB; Van Cutsem, E; Rougier, P; ESMO Guidelines Working, Group (October 2012). "Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 23 Suppl 7: vii33-40. doi:10.1093/annonc/mds224. PMID 22997452.
{{cite journal}}:|access-date=requires|url=(help) - ^ a b Bussom S, Saif MW (5 March 2010). "Methods and rationale for the early detection of pancreatic cancer. Highlights from the "2010 ASCO Gastrointestinal Cancers Symposium". Orlando, FL, USA. January 22-24, 2010". JOP : Journal of the pancreas. 11 (2): 128–30. PMID 20208319.
- ^ Shahrokni A, Saif MW (10 July 2013). "Metastatic pancreatic cancer: the dilemma of quality vs. quantity of life". JOP : Journal of the pancreas. 14 (4): 391–4. PMID 23846935.
- ^ Bardou M, Le Ray I (December 2013). "Treatment of pancreatic cancer: A narrative review of cost-effectiveness studies". Best practice & research. Clinical gastroenterology. 27 (6): 881–92. doi:10.1016/j.bpg.2013.09.006. PMID 24182608.
- ^ Hariharan D, Saied A, Kocher HM (2008). "Analysis of mortality rates for pancreatic cancer across the world". HPB. 10 (1): 58–62. doi:10.1080/13651820701883148. PMC 2504856. PMID 18695761.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "American Cancer Society: Cancer Facts & Figures 2010: see page 4 for incidence estimates, and page 19 for survival percentages" (PDF).
- ^ a b c "Pancreatic Cancer Treatment (PDQ®) Health Professional Version". NCI. 21 February 2014. Retrieved 8 June 2014.
- ^ Hardison, Brooke Layne (23 April 2010). "The Financial Burden of Cancer". NCI. Retrieved 8 June 2014.
- ^ Öberg, K; Knigge, U; Kwekkeboom, D; Perren, A; ESMO Guidelines Working, Group (2012 Oct). "Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 23 Suppl 7: vii124-30. PMID 22997445.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help) - ^ Tobias Jeffrey S., Hochhauser, Daniel, Cancer and its Management, p. 276, 2010 (6th edn), ISBN 1118713257, 9781118713259
- ^ a b c Johns Hopkins Medicine; The Sol Goldman Pancreas Cancer Research Center. Types of Pancreas Tumors. http://pathology.jhu.edu/pancreas/BasicTypes1.php
- ^ Farrell, JJ; Fernández-del Castillo, C (June 2013). "Pancreatic cystic neoplasms: management and unanswered questions". Gastroenterology. 144 (6): 1303–15. doi:10.1053/j.gastro.2013.01.073. PMID 23622140.
- ^ Burns, WR; Edil, BH (2012 Mar). "Neuroendocrine pancreatic tumors: guidelines for management and update". Current treatment options in oncology. 13 (1): 24–34. PMID 22198808.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help) - ^ Öberg, K; Knigge, U; Kwekkeboom, D; Perren, A; ESMO Guidelines Working, Group (2012 Oct). "Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 23 Suppl 7: vii124-30. PMID 22997445.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help) - ^ Burns, WR; Edil, BH (2012 Mar). "Neuroendocrine pancreatic tumors: guidelines for management and update". Current treatment options in oncology. 13 (1): 24–34. PMID 22198808.
{{cite journal}}:|access-date=requires|url=(help); Check date values in:|date=(help) - ^ a b c Vincent, A; Herman, J; Schulick, R; Hruban, RH; Goggins, M (13 August 2011). "Pancreatic cancer". Lancet. 378 (9791): 607–20. doi:10.1016/S0140-6736(10)62307-0. PMID 21620466.
- ^ Tobias Jeffrey S., Hochhauser, Daniel, Cancer and its Management, 2010 (6th edn), pp. 276-277, ISBN 1118713257, 9781118713259
- ^ a b c d e Bond-Smith, G; Banga, N; Hammond, TM; Imber, CJ (16 May 2012). "Pancreatic adenocarcinoma". BMJ (Clinical research ed.). 344: e2476. doi:10.1136/bmj.e2476. PMID 22592847.
- ^ a b "What You Need To Know About Cancer of the Pancreas — National Cancer Institute". 16 September 2002. p. 4/5. Retrieved 22 December 2007.
- ^ Dragovich, Tomislav (13 September 2011). "Pancreatic Cancer". Medscape Reference.
- ^ a b Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 328. doi:10.3322/caac.21190. PMID 23856911.
- ^ Pannala R, Basu A, Petersen GM, Chari ST (January 2009). "New-onset Diabetes: A Potential Clue to the Early Diagnosis of Pancreatic Cancer". The Lancet Oncology. 10 (1): 88–95. doi:10.1016/S1470-2045(08)70337-1. PMC 2795483. PMID 19111249.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 342–343. doi:10.3322/caac.21190. PMID 23856911.
- ^ Medscape > Pancreatic Cancer Author: Tomislav Dragovich. Chief Editor: Jules E Harris. Updated: 5 May 2011
- ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 329. doi:10.3322/caac.21190. PMID 23856911.
- ^ AJCC Cancer Staging Manual 2nd edition; Chapter 15; Pancreas – original pages 95–98; page 95 for citation regarding "...lesser degree of involvement of bones and brain and other anatomical sites." http://www.cancerstaging.org/products/csmanual2ed.pdf
- ^ "Causes of pancreatic cancer". NHS Choices. National Health Service, England. Retrieved 9 June 2014.
- ^ "ACS :: What Are the Risk Factors for Cancer of the Pancreas?". Archived from the original on 12 October 2007. Retrieved 13 December 2007.
- ^ Bosetti, C; Lucenteforte, E; Silverman, DT; Petersen, G; Bracci, PM; Ji, BT; Negri, E; Li, D; Risch, HA; Olson, SH; Gallinger, S; Miller, AB; Bueno-de-Mesquita, HB; Talamini, R; Polesel, J; Ghadirian, P; Baghurst, PA; Zatonski, W; Fontham, E; Bamlet, WR; Holly, EA; Bertuccio, P; Gao, YT; Hassan, M; Yu, H; Kurtz, RC; Cotterchio, M; Su, J; Maisonneuve, P; Duell, EJ; Boffetta, P; La Vecchia, C (July 2012). "Cigarette smoking and pancreatic cancer: an analysis from the International Pancreatic Cancer Case-Control Consortium (Panc4)". Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 23 (7): 1880–8. PMID 22104574.
{{cite journal}}:|access-date=requires|url=(help) - ^ Lowenfels AB, Maisonneuve P, DiMagno EP; et al. (March 1997). "Hereditary pancreatitis and the risk of pancreatic cancer. International Hereditary Pancreatitis Study Group". J. Natl. Cancer Inst. 89 (6): 442–6. doi:10.1093/jnci/89.6.442. PMID 9091646.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Howes N, Lerch MM, Greenhalf W; et al. (March 2004). "Clinical and genetic characteristics of hereditary pancreatitis in Europe". Clin. Gastroenterol. Hepatol. 2 (3): 252–61. PMID 15017610.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Rebours V, Boutron-Ruault MC, Schnee M; et al. (January 2008). "Risk of pancreatic adenocarcinoma in patients with hereditary pancreatitis: a national exhaustive series". Am. J. Gastroenterol. 103 (1): 111–9. doi:10.1111/j.1572-0241.2007.01597.x. PMID 18184119.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Greenhalf W, Grocock C, Harcus M, Neoptolemos J (2009). "Screening of high-risk families for pancreatic cancer". Pancreatology. 9 (3): 215–22. doi:10.1159/000210262. PMID 19349734.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 322–323. doi:10.3322/caac.21190. PMID 23856911.
- ^ a b c Ghaneh P, Costello E, Neoptolemos JP (August 2007). "Biology and management of pancreatic cancer". Gut. 56 (8): 1134–52. doi:10.1136/gut.2006.103333. PMC 1955499. PMID 17625148.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Efthimiou E, Crnogorac-Jurcevic T, Lemoine NR, Brentnall TA (February 2001). "Inherited predisposition to pancreatic cancer". Gut. 48 (2): 143–7. doi:10.1136/gut.48.2.143. PMC 1728218. PMID 11156628.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Raderer M, Wrba F, Kornek G, Maca T, Koller DY, Weinlaender G, Hejna M, Scheithauer W (1998). "Association between Helicobacter pylori Infection and Pancreatic Cancer". Oncology. 55 (1): 16–19. doi:10.1159/000011830. PMID 9428370.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, Perez-Perez G, Taylor PR, Virtamo J, Albanes D (June 2001). "Helicobacter pylori seropositivity as a risk factor for pancreatic cancer". J. Natl. Cancer Inst. 93 (12): 937–41. doi:10.1093/jnci/93.12.937. PMID 11416115.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Michaud DS, Joshipura K, Giovannucci E, Fuchs CS (January 2007). "A prospective study of periodontal disease and pancreatic cancer in US male health professionals". Journal of the National Cancer Institute. 99 (2): 171–5. doi:10.1093/jnci/djk021. PMID 17228001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Chan JM, Wang F, Holly EA (September 2005). "Vegetable and fruit intake and pancreatic cancer in a population-based case-control study in the San Francisco bay area". Cancer Epidemiology, Biomarkers & Prevention. 14 (9): 2093–7. doi:10.1158/1055-9965.EPI-05-0226. PMID 16172215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Larsson SC, Wolk A (January 2012). "Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies". Br J Cancer. Online first (3): 603–7. doi:10.1038/bjc.2011.585. PMC 3273353. PMID 22240790.
- ^ "Soft Drink and Juice Consumption and Risk of Pancreatic Cancer: The Singapore Chinese Health Study".
- ^ Liu H, Huang D, McArthur DL, Boros LG, Nissen N, Heaney AP (2010). "Fructose Induces Transketolase Flux to Promote Pancreatic Cancer Growth". Cancer Res. 70 (15): 6368–76. doi:10.1158/0008-5472.CAN-09-4615. PMID 20647326. Retrieved 25 October 2013.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ van Rees BP, Tascilar M, Hruban RH, Giardiello FM, Tersmette AC, Offerhaus GJ (1999). "Remote partial gastrectomy as a risk factor for pancreatic cancer: potential for preventive strategies". Ann Oncol. 10 Suppl 4: 204–207. PMID 10436823.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tersmette AC, Giardiello FM, Tytgat GN, Offerhaus GJ (1995). "Carcinogenesis after remote peptic ulcer surgery: the long-term prognosis of partial gastrectomy". Scand J Gastroenterol Suppl. 212: 96–99. doi:10.3109/00365529509090306. PMID 8578237.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ National Institute on Alcohol Abuse and Alcoholism Alcohol and Cancer - Alcohol Alert No. 21-1993
- ^ Villeneuve PJ, Johnson KC, Hanley AJ, Mao Y (February 2000). "Alcohol, tobacco and coffee consumption and the risk of pancreatic cancer: results from the Canadian Enhanced Surveillance System case-control project. Canadian Cancer Registries Epidemiology Research Group". European Journal of Cancer Prevention. 9 (1): 49–58. doi:10.1097/00008469-200002000-00007. PMID 10777010.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Cancer Research UK Pancreatic cancer risks and causes
- ^ Bakkevold KE, Arnesjø B, Kambestad B (April 1992). "Carcinoma of the pancreas and papilla of Vater: presenting symptoms, signs, and diagnosis related to stage and tumour site. A prospective multicentre trial in 472 patients. Norwegian Pancreatic Cancer Trial". Scandinavian Journal of Gastroenterology. 27 (4): 317–25. doi:10.3109/00365529209000081. PMID 1589710.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Frank J. Domino M.D. (2007). 5 minutes clinical suite version 3. Philadelphia, PA: Lippincott Williams & Wilkins.[page needed]
- ^ a b Philip Agop, "Pancreatic Cancer". ACP PIER & AHFX DI Essentials. American College of Physicians. 4 Apr 2008. Accessed 7 Apr 2009.[page needed]
- ^ Tempero MA, Arnoletti JP, Behrman S, Ben-Josef E, Benson AB, Berlin JD, Cameron JL, Casper ES, Cohen SJ, Duff M, Ellenhorn JD, Hawkins WG, Hoffman JP, Kuvshinoff BW, Malafa MP, Muscarella P, Nakakura EK, Sasson AR, Thayer SP, Tyler DS, Warren RS, Whiting S, Willett C, Wolff RA (September 2010). "Pancreatic adenocarcinoma". J Natl Compr Canc Netw. 8 (9): 972–1017. PMC 3135380. PMID 20876541.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 329–330. doi:10.3322/caac.21190. PMID 23856911.
- ^ "Pancreatic cancer survival by stage". American Cancer Society. Retrieved 29 September 2014.
- ^ Sarkar FH, Banerjee S, Li Y (November 2007). "Pancreatic cancer: pathogenesis, prevention and treatment". Toxicol. Appl. Pharmacol. 224 (3): 326–36. doi:10.1016/j.taap.2006.11.007. PMC 2094388. PMID 17174370.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 324. doi:10.3322/caac.21190. PMID 23856911.
- ^ Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, Maitra A, Pollack JR (31 January 2012). "Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer". Proceedings of the National Academy of Sciences of the United States of America. 109 (5): E252–9. doi:10.1073/pnas.1114817109. PMC 3277150. PMID 22233809.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW (26 September 2008). "Core signaling pathways in human pancreatic cancers revealed by global genomic analyses". Science. 321 (5897): 1801–6. doi:10.1126/science.1164368. PMC 2848990. PMID 18772397.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Delpu Y, Hanoun N, Lulka H, Sicard F, Selves J, Buscail L, Torrisani J, Cordelier P (2011). "Genetic and epigenetic alterations in pancreatic carcinogenesis". Curr Genomics. 12 (1): 15–24. doi:10.2174/138920211794520132. PMC 3129039. PMID 21886451.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f g Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, Hassan M, Evans DB (2007). "Population-Based Study of Islet Cell Carcinoma". Annals of Surgical Oncology. 14 (12): 3492–3500. doi:10.1245/s10434-007-9566-6. PMC 2077912. PMID 17896148.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Benson AB, Myerson RJ, and Sasson AR. Pancreatic, Neuroendocrine GI, and Adrenal Cancers. Cancer Management 13th edition. http://www.cancernetwork.com/cancer-management/pancreatic/article/10165/1802606
- ^ "The prognosis of patients with PENs is difficult to predict, in part because the definition of malignancy in PENs has been ambiguous. By some, PENs have been defined as malignant only when lymph nodes are involved or liver metastases are documented. Other investigators have included vascular invasion or invasion of adjacent structures as evidence of malignancy. However, the concept that a PEN removed successfully without recurrence was therefore biologically benign could be challenged. In fact, strict separation of PENs into benign and malignant groups may be less clinically useful than the definition of prognostic factors."Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS (2002). "Prognostic Factors in Pancreatic Endocrine Neoplasms: An Analysis of 136 Cases with a Proposal for Low-Grade and Intermediate-Grade Groups". Journal of Clinical Oncology. 20 (11): 2633–2642. doi:10.1200/JCO.2002.10.030. PMID 12039924.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "One of the most controversial aspects of PENs has been the prediction of prognosis."Klimstra DS (2007). "Nonductal neoplasms of the pancreas". Modern Pathology. 20: S94–S112. doi:10.1038/modpathol.3800686. PMID 17486055.
- ^ "The classification of these tumors remains controversial, and prognosis is difficult to predict" Wendy L. Frankel (2006) Update on Pancreatic Endocrine Tumors. Archives of Pathology & Laboratory Medicine: July 2006, Vol. 130, No. 7, pp. 963–966. http://www.archivesofpathology.org/doi/full/10.1043/1543-2165(2006)130[963:UOPET]2.0.CO;2
- ^ Modlin http://onlinelibrary.wiley.com/doi/10.1002/cncr.11105/pdf
- ^ Coughlin SS, Calle EE, Patel AV, Thun MJ (December 2000). "Predictors of pancreatic cancer mortality among a large cohort of United States adults". Cancer Causes & Control. 11 (10): 915–23. doi:10.1023/A:1026580131793. ISSN 0957-5243. PMID 11142526.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Zheng W, McLaughlin JK, Gridley G, Bjelke E, Schuman LM, Silverman DT, Wacholder S, Co-Chien HT, Blot WJ, Fraumeni JF (September 1993). "A cohort study of smoking, alcohol consumption, and dietary factors for pancreatic cancer (United States)". Cancer Causes & Control. 4 (5): 477–82. doi:10.1007/BF00050867. PMID 8218880.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Larsson SC, Håkansson N, Näslund I, Bergkvist L, Wolk A (February 2006). "Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study". Cancer Epidemiology, Biomarkers & Prevention. 15 (2): 301–05. doi:10.1158/1055-9965.EPI-05-0696. PMID 16492919.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schernhammer E, Wolpin B, Rifai N, Cochrane B, Manson JA, Ma J, Giovannucci E, Thomson C, Stampfer MJ, Fuchs C (June 2007). "Plasma folate, vitamin B6, vitamin B12, and homocysteine and pancreatic cancer risk in four large cohorts". Cancer Research. 67 (11): 5553–60. doi:10.1158/0008-5472.CAN-06-4463. PMID 17545639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW (January 2011). "Effect of daily aspirin on long term risk of death due to cancer: analysis of individual patient data from randomised trials". Lancet. 337 (9759): 31–41. doi:10.1016/S0140-6736(10)62110-1. PMID 21144578.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Stoita, A; Penman, ID; Williams, DB (21 May 2011). "Review of screening for pancreatic cancer in high risk individuals". World journal of gastroenterology : WJG. 17 (19): 2365–71. doi:10.3748/wjg.v17.i19.2365. PMC 3103788. PMID 21633635.
{{cite journal}}:|access-date=requires|url=(help)CS1 maint: unflagged free DOI (link) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 329–332. doi:10.3322/caac.21190. PMID 23856911.
- ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 332–343. doi:10.3322/caac.21190. PMID 23856911.
- ^ "Surgical Treatment of Pancreatic Cancer". Johns Hopkins University. Retrieved 5 September 2009.
- ^ Corbo V, Tortora G, Scarpa A (2012) Molecular pathology of pancreatic cancer: from bench-to-bedside translation. Curr Drug Targets
- ^ Allen, VB; Gurusamy, KS; Takwoingi, Y; Kalia, A; Davidson, BR (2013 Nov 25). "Diagnostic accuracy of laparoscopy following computed tomography (CT) scanning for assessing the resectability with curative intent in pancreatic and periampullary cancer". The Cochrane database of systematic reviews. 11: CD009323. PMID 24272022.
{{cite journal}}: Check date values in:|date=(help) - ^ Wolfgang, CL; Herman, JM; Laheru, DA; Klein, AP; Erdek, MA; Fishman, EK; Hruban, RH (September 2013). "Recent progress in pancreatic cancer". CA: a cancer journal for clinicians. 63 (5): 336–337. doi:10.3322/caac.21190. PMID 23856911.
- ^ Arcidiacono PG, Calori G, Carrara S, McNicol ED, Testoni PA (2011). Arcidiacono, Paolo G (ed.). "Celiac plexus block for pancreatic cancer pain in adults". Cochrane Database Syst Rev (3): CD007519. doi:10.1002/14651858.CD007519.pub2. PMID 21412903.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Büchler MW (March 2004). "A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer". The New England Journal of Medicine. 350 (12): 1200–10. doi:10.1056/NEJMoa032295. PMID 15028824.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Thota, R; Pauff, JM; Berlin, JD (January 2014). "Treatment of metastatic pancreatic adenocarcinoma: a review". Oncology (Williston Park, N.Y.). 28 (1): 70–4. PMID 24683721.
- ^ "Palliative or Supportive Care". American Cancer Society. Retrieved 20 August 2014.
- ^ Buanes, TA (14 August 2014). "Pancreatic cancer-improved care achievable". World journal of gastroenterology : WJG. 20 (30): 10405–10418. PMID 25132756.
- ^ "If treatment for pancreatic cancer stops workin". American Cancer Society. Retrieved 20 August 2014.
- ^ Fazal, S; Saif, MW (10 March 2007). "Supportive and palliative care of pancreatic cancer". JOP : Journal of the pancreas. 8 (2): 240–53. PMID 17356251.
- ^ "Pancreatic cancer survival by stage". American Cancer Society. Retrieved 29 September 2014.
- ^ "Pancreatic cancer survival by stage". American Cancer Society. Retrieved 29 September 2014.
- ^ "WHO Disease and injury country estimates". World Health Organization. 2009. Retrieved 11 November 2009.
- ^ Lozano R; et al. (15 December 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ "Pancreatic Cancer — National Cancer Institute, U.S. National Institutes of Health (Accessed 28 April 2011)". Cancer.gov. Retrieved 15 September 2009.
- ^ Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007). "Cancer statistics, 2007". CA. 57 (1): 43–66. doi:10.3322/canjclin.57.1.43. PMID 17237035.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "What's new in pancreatic cancer research and treatment?". American Cancer Society. Retrieved 17 July 2014.
- ^ "Pancreatic cancer research". Cancer Research UK. Retrieved 17 July 2014.
- ^ Marie, McCulloch (13 July 2014). "Researchers step up hunt for new pancreatic-cancer treatments". The Philadelphia Inquirer. Retrieved 17 July 2014.
- ^ Australian Pancreatic Genome Initiative http://www.pancreaticcancer.net.au/. Retrieved 17 July 2014.
{{cite web}}: Missing or empty|title=(help) - ^ Biankin, Andrew V.; Waddell, Nicola; Kassahn, Karin S.; Gingras, Marie-Claude; Muthuswamy, Lakshmi B.; Johns, Amber L.; Miller, David K.; Wilson, Peter J.; Patch, Ann-Marie; Wu, Jianmin; Chang, David K.; Cowley, Mark J.; Gardiner, Brooke B.; Song, Sarah; Harliwong, Ivon; Idrisoglu, Senel; Nourse, Craig; Nourbakhsh, Ehsan; Manning, Suzanne; Wani, Shivangi; Gongora, Milena; Pajic, Marina; Scarlett, Christopher J.; Gill, Anthony J.; Pinho, Andreia V.; Rooman, Ilse; Anderson, Matthew; Holmes, Oliver; Leonard, Conrad; Taylor, Darrin; Wood, Scott; Xu, Qinying; Nones, Katia; Lynn Fink, J.; Christ, Angelika; Bruxner, Tim; Cloonan, Nicole; Kolle, Gabriel; Newell, Felicity; Pinese, Mark; Scott Mead, R.; Humphris, Jeremy L.; Kaplan, Warren; Jones, Marc D.; Colvin, Emily K.; Nagrial, Adnan M.; Humphrey, Emily S.; Chou, Angela; Chin, Venessa T.; Chantrill, Lorraine A.; Mawson, Amanda; Samra, Jaswinder S.; Kench, James G.; Lovell, Jessica A.; Daly, Roger J.; Merrett, Neil D.; Toon, Christopher; Epari, Krishna; Nguyen, Nam Q.; Barbour, Andrew; Zeps, Nikolajs; Biankin, Andrew V.; Johns, Amber L.; Mawson, Amanda; Chang, David K.; Scarlett, Christopher J.; Brancato, Mary-Anne L.; Rowe, Sarah J.; Simpson, Skye L.; Martyn-Smith, Mona; Thomas, Michelle T.; Chantrill, Lorraine A.; Chin, Venessa T.; Chou, Angela; Cowley, Mark J.; Humphris, Jeremy L.; Jones, Marc D.; Scott Mead, R.; Nagrial, Adnan M.; Pajic, Marina; Pettit, Jessica; Pinese, Mark; Rooman, Ilse; Wu, Jianmin; Tao, Jiang; DiPietro, Renee; Watson, Clare; Wong, Rachel; Pinho, Andreia V.; Giry-Laterriere, Marc; Daly, Roger J.; Musgrove, Elizabeth A.; Sutherland, Robert L.; Grimmond, Sean M.; Waddell, Nicola; Kassahn, Karin S.; Miller, David K.; Wilson, Peter J.; Patch, Ann-Marie; Song, Sarah; Harliwong, Ivon; Idrisoglu, Senel; Nourse, Craig; Nourbakhsh, Ehsan; Manning, Suzanne; Wani, Shivangi; Gongora, Milena; Anderson, Matthew; Holmes, Oliver; Leonard, Conrad; Taylor, Darrin; Wood, Scott; Xu, Qinying; Nones, Katia; Lynn Fink, J.; Christ, Angelika; Bruxner, Tim; Cloonan, Nicole; Newell, Felicity; Pearson, John V.; Samra, Jaswinder S.; Gill, Anthony J.; Pavlakis, Nick; Guminski, Alex; Toon, Christopher; Biankin, Andrew V.; Asghari, Ray; Merrett, Neil D.; Chang, David K.; Pavey, Darren A.; Das, Amitabha; Cosman, Peter H.; Ismail, Kasim; O’Connor, Chelsie; Lam, Vincent W.; McLeod, Duncan; Pleass, Henry C.; Richardson, Arthur; James, Virginia; Kench, James G.; Cooper, Caroline L.; Joseph, David; Sandroussi, Charbel; Crawford, Michael; Gallagher, James; Texler, Michael; Forrest, Cindy; Laycock, Andrew; Epari, Krishna P.; Ballal, Mo; Fletcher, David R.; Mukhedkar, Sanjay; Spry, Nigel A.; DeBoer, Bastiaan; Chai, Ming; Zeps, Nikolajs; Beilin, Maria; Feeney, Kynan; Nguyen, Nam Q.; Ruszkiewicz, Andrew R.; Worthley, Chris; Tan, Chuan P.; Debrencini, Tamara; Chen, John; Brooke-Smith, Mark E.; Papangelis, Virginia; Tang, Henry; Barbour, Andrew P.; Clouston, Andrew D.; Martin, Patrick; O’Rourke, Thomas J.; Chiang, Amy; Fawcett, Jonathan W.; Slater, Kellee; Yeung, Shinn; Hatzifotis, Michael; Hodgkinson, Peter; Christophi, Christopher; Nikfarjam, Mehrdad; Mountain Victorian Cancer Biobank, Angela; Eshleman, James R.; Hruban, Ralph H.; Maitra, Anirban; Iacobuzio-Donahue, Christine A.; Schulick, Richard D.; Wolfgang, Christopher L.; Morgan, Richard A.; Hodgin, Mary B.; Scarpa, Aldo; Lawlor, Rita T.; Capelli, Paola; Beghelli, Stefania; Corbo, Vincenzo; Scardoni, Maria; Pederzoli, Paolo; Tortora, Giampaolo; Bassi, Claudio; Tempero, Margaret A.; Kakkar, Nipun; Zhao, Fengmei; Qing Wu, Yuan; Wang, Min; Muzny, Donna M.; Fisher, William E.; Charles Brunicardi, F.; Hodges, Sally E.; Reid, Jeffrey G.; Drummond, Jennifer; Chang, Kyle; Han, Yi; Lewis, Lora R.; Dinh, Huyen; Buhay, Christian J.; Beck, Timothy; Timms, Lee; Sam, Michelle; Begley, Kimberly; Brown, Andrew; Pai, Deepa; Panchal, Ami; Buchner, Nicholas; De Borja, Richard; Denroche, Robert E.; Yung, Christina K.; Serra, Stefano; Onetto, Nicole; Mukhopadhyay, Debabrata; Tsao, Ming-Sound; Shaw, Patricia A.; Petersen, Gloria M.; Gallinger, Steven; Hruban, Ralph H.; Maitra, Anirban; Iacobuzio-Donahue, Christine A.; Schulick, Richard D.; Wolfgang, Christopher L.; Morgan, Richard A.; Lawlor, Rita T.; Capelli, Paola; Corbo, Vincenzo; Scardoni, Maria; Tortora, Giampaolo; Tempero, Margaret A.; Mann, Karen M.; Jenkins, Nancy A.; Perez-Mancera, Pedro A.; Adams, David J.; Largaespada, David A.; Wessels, Lodewyk F. A.; Rust, Alistair G.; Stein, Lincoln D.; Tuveson, David A.; Copeland, Neal G.; Musgrove, Elizabeth A.; Scarpa, Aldo; Eshleman, James R.; Hudson, Thomas J.; Sutherland, Robert L.; Wheeler, David A.; Pearson, John V.; McPherson, John D.; Gibbs, Richard A.; Grimmond, Sean M. (24 October 2012). "Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes". Nature. 491 (7424): 399–405. doi:10.1038/nature11547.
- ^ European Registry of Hereditary Pancreatitis and Familial Pancreatic Cancer (EUROPAC) website http://www.europac-org.eu/. Retrieved 17 July 2014.
{{cite web}}: Missing or empty|title=(help) - ^ Moore, M. J.; Goldstein, D.; Hamm, J.; Figer, A.; Hecht, J. R.; Gallinger, S.; Au, H. J.; Murawa, P.; Walde, D.; Wolff, R. A.; Campos, D.; Lim, R.; Ding, K.; Clark, G.; Voskoglou-Nomikos, T.; Ptasynski, M.; Parulekar, W. (9 April 2007). "Erlotinib Plus Gemcitabine Compared With Gemcitabine Alone in Patients With Advanced Pancreatic Cancer: A Phase III Trial of the National Cancer Institute of Canada Clinical Trials Group". Journal of Clinical Oncology. 25 (15): 1960–1966. doi:10.1200/JCO.2006.07.9525.
- ^ Neesse, Albrecht; Gress, Thomas M.; Tuveson, David A.; Michl, Patrick; Krug, Sebastian. "Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma". OncoTargets and Therapy: 33. doi:10.2147/OTT.S38111.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cook, N; Jodrell, DI; Tuveson, DA (March 2012). "Predictive in vivo animal models and translation to clinical trials". Drug Discovery Today. 17 (5–6): 253–60. doi:10.1016/j.drudis.2012.02.003. PMID 22493784.
- ^ Fong, Yuman; Ady, Justin; Heffner, Jacqueline; Klein, Elizabeth. "Oncolytic viral therapy for pancreatic cancer: current research and future directions". Oncolytic Virotherapy: 35. doi:10.2147/OV.S53858.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Subar, D.; Gobardhan, P.D.; Gayet, B. "Laparoscopic pancreatic surgery". Best Practice & Research Clinical Gastroenterology. 28 (1): 123–132. doi:10.1016/j.bpg.2013.11.011.
External links









