Dimercaprol
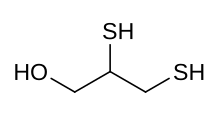 | |
 Skeletal formula and ball and stick model of dimercaprol | |
| Clinical data | |
|---|---|
| Trade names | BAL in Oil |
| Other names | 2,3-Dimercaptopropanol British Anti-Lewisite 2,3-Dithiopropanol 2,3-Dimercaptopropan-1-ol British antilewisite |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Urine[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.394 |
| Chemical and physical data | |
| Formula | C3H8OS2 |
| Molar mass | 124.22 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.239 g/cm3 |
| Boiling point | 393 °C (739 °F) at 2.0 kPa |
| |
| |
Dimercaprol, also called British anti-Lewisite (BAL), is a medication used to treat acute poisoning by arsenic, mercury, gold, and lead.[3] It may also be used for antimony, thallium, or bismuth poisoning, although the evidence for those uses is not very strong.[3][4] It is given by injection into a muscle.[3]
Common side effects include high blood pressure, pain at the site of the injection, vomiting, and fever.[3] It is not recommended for people with peanut allergies as it is typically formulated as a suspension in peanut oil.[3] It is unclear if use in pregnancy is safe for the baby.[3] Dimercaprol is a chelator and works by binding with heavy metals.[3] It has a very pungent odor.
Dimercaprol was first made during World War II.[5] It is on the World Health Organization's List of Essential Medicines.[6]
Medical uses
[edit]Dimercaprol has long been the mainstay of chelation therapy for lead or arsenic poisoning,[7] and it is an essential drug.[6] It is also used as an antidote to the chemical weapon Lewisite. Nonetheless, because it can have serious adverse effects, researchers have also pursued development of less toxic analogues,[7] such as succimer.
Wilson's disease is a genetic disorder in which copper builds up inside the liver and other tissues. Dimercaprol is a copper chelating agent that has been approved by the FDA to treat Wilson's disease.[8]
Dimercaprol also shows effectiveness against snakebite by potently antagonizing the activity of Zn2+-dependent snake venom metalloproteinases in vitro.[9]
Mechanism of action
[edit]Arsenic and some other heavy metals act by chelating with adjacent thiol residues on metabolic enzymes, creating a chelate complex that inhibits the affected enzyme's activity.[10] Dimercaprol competes with the thiol groups for binding the metal ion, which is then excreted in the urine.[citation needed]
Dimercaprol is itself toxic, with a narrow therapeutic range and a tendency to concentrate arsenic in some organs. Other drawbacks include the need to administer it by painful intramuscular injection[11] Serious side effects include nephrotoxicity and hypertension.
Dimercaprol has been found to form stable chelates in vivo with many other metals including inorganic mercury, antimony, bismuth, cadmium, chromium, cobalt, gold, and nickel. However, it is not necessarily the treatment of choice for toxicity to these metals. Dimercaprol has been used as an adjunct in the treatment of the acute encephalopathy of lead toxicity. It is a potentially toxic drug, and its use may be accompanied by multiple side effects. Although treatment with dimercaprol will increase the excretion of cadmium, there is a concomitant increase in renal cadmium concentration, so that its use in case of cadmium toxicity is to be avoided. It does, however, remove inorganic mercury from the kidneys; but is not useful in the treatment of alkylmercury or phenylmercury toxicity. Dimercaprol also enhances the toxicity of selenium and tellurium, so it is not to be used to remove these elements from the body.[citation needed]
History
[edit]The original name of dimercaprol reflects its origins as a compound secretly developed by British biochemists at Oxford University in the beginning of the World War II, with the first synthesis in July 1940[12][13] as an antidote for lewisite, a now-obsolete arsenic-based chemical warfare agent.[12]
See also
[edit]- 2,3-Dimercapto-1-propanesulfonic acid
- Dimercaptosuccinic acid
- DMSA scan
- EDTA
- Heavy metal poisoning
- Penicillamine
References
[edit]- ^ Poisoning in Children. Jaypee Brothers Publishers. 2013. p. 70. ISBN 978-93-5025-773-9.
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 697. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The prefixes 'mercapto' (–SH), and 'hydroseleno' or selenyl (–SeH), etc. are no longer recommended.
- ^ a b c d e f g "Dimercaprol". The American Society of Health-System Pharmacists. Archived from the original on 21 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 62. hdl:10665/44053. ISBN 978-92-4-154765-9.
- ^ Greenwood D (2008). "Antiprotozoal Agents". Antimicrobial Drugs: Chronicle of a Twentieth Century Medical Triumph. OUP Oxford. p. 281. ISBN 978-0-19-953484-5. Archived from the original on 2016-12-20.
- ^ a b World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b Flora SJ, Pachauri V (July 2010). "Chelation in metal intoxication". International Journal of Environmental Research and Public Health. 7 (7): 2745–2788. doi:10.3390/ijerph7072745. PMC 2922724. PMID 20717537.
- ^ Leggio L, Addolorato G, Abenavoli L, Gasbarrini G (2005). "Wilson's disease: clinical, genetic and pharmacological findings". International Journal of Immunopathology and Pharmacology. 18 (1): 7–14. doi:10.1177/039463200501800102. PMID 15698506. S2CID 26059921.
- ^ Albulescu LO, Hale MS, Ainsworth S, Alsolaiss J, Crittenden E, Calvete JJ, et al. (May 2020). "Preclinical validation of a repurposed metal chelator as an early-intervention therapeutic for hemotoxic snakebite". Science Translational Medicine. 12 (542). doi:10.1126/scitranslmed.aay8314. PMC 7116364. PMID 32376771.
- ^ Goldman M, Dacre JC (1989). "Lewisite: Its Chemistry, Toxicology, and Biological Effects". Reviews of Environmental Contamination and Toxicology. Vol. 110. pp. 75–115. doi:10.1007/978-1-4684-7092-5_2. ISBN 978-1-4684-7094-9. PMID 2692088.
- ^ Mückter H, Liebl B, Reichl FX, Hunder G, Walther U, Fichtl B (August 1997). "Are we ready to replace dimercaprol (BAL) as an arsenic antidote?". Human & Experimental Toxicology. 16 (8): 460–465. Bibcode:1997HETox..16..460M. doi:10.1177/096032719701600807. PMID 9292286. S2CID 44772701.
- ^ a b Tabangcura Jr D, Daubert GP. "British anti-Lewisite". Archived from the original on 2009-02-02.
- ^ Peters RA, Stocken LA, Thompson RH (1945). "British anti-lewisite (BAL)". Nature. 156 (3969): 616–619. Bibcode:1945Natur.156..616P. doi:10.1038/156616a0. PMID 21006485. S2CID 4129186.
External links
[edit]- "Dimercaprol". Drug Information Portal. U.S. National Library of Medicine.
