Barium
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Barium | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈbɛəriəm/ | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray; with a pale yellow tint[1] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Ba) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Barium in the periodic table | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic number (Z) | 56 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Group | group 2 (alkaline earth metals) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Period | period 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Block | s-block | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Xe] 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 18, 8, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1000 K (727 °C, 1341 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 2118 K (1845 °C, 3353 °F) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Density (at 20° C) | 3.594 g/cm3 [4] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 3.338 g/cm3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 7.12 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 142 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar heat capacity | 28.07 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +1, +2 (a strongly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 0.89 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 222 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 215±11 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 268 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic (bcc) (cI2) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Lattice constant | a = 502.5 pm (at 20 °C)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | 20.47×10−6/K (at 20 °C)[4] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 18.4 W/(m⋅K) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | 332 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic[5] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | +20.6×10−6 cm3/mol[6] | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Young's modulus | 13 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 4.9 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 9.6 GPa | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound thin rod | 1620 m/s (at 20 °C) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 1.25 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Number | 7440-39-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Discovery | Carl Wilhelm Scheele (1772) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| First isolation | Humphry Davy (1808) | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes of barium | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Barium (/[invalid input: 'icon']ˈbɛəriəm/ BAIR-ee-əm) is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in Group 2, a soft silvery metallic alkaline earth metal. [citation needed] Barium is never found in nature in its pure form due to its reactivity with air. Its oxide is historically known as baryta but it reacts with water and carbon dioxide and is not found as a mineral. The most common naturally occurring minerals are the very insoluble barium sulfate, BaSO4 (barite), and barium carbonate, BaCO3 (witherite). Barium's name originates from Greek barys (βαρύς), meaning "heavy", describing the high density of some common barium-containing ores.
Barium has few industrial applications, but the metal has been historically used to scavenge air in vacuum tubes. Barium compounds impart a green color to flames and have been used in fireworks. Barium sulfate is used for its density, insolubility, and X-ray opacity. It is used as an insoluble heavy additive to oil well drilling mud, and in purer form, as an X-ray radiocontrast agent for imaging the human gastrointestinal tract. Soluble barium compounds are poisonous due to release of the soluble barium ion, and have been used as rodenticides. New uses for barium continue to be sought. It is a component of some "high temperature" YBCO superconductors, and electroceramics.
Characteristics

Physical properties
Barium is a soft, silvery white alkali earth metal, which quickly oxidizes in air.[8] It crystallizes in body centered cubic lattices. It burns with a green to pale green flame, resulting from emission at 524.2 and 513.7 nm. Its simple compounds are notable for their relatively high (for an alkaline earth element) specific gravity. This high density is true of the most common barium-bearing mineral, barite (BaSO4), also called 'heavy spar' due to the high density (4.5 g/cm³).
Chemical properties
Barium, as for other alkali earth (group II) metals, is highly reducing. It reacts exothermically with oxygen at room temperature to form barium oxide and peroxide. Because of its sensitivity to air, samples are generally stored under protective oils. The reaction is violent if barium is powdered. The metal is readily attacked in most acids, with the notable exception of sulfuric acid, as passivation stops the reaction by forming the insoluble barium sulfate. It also reacts violently with water according to the reaction:
- Ba + 2 H2O → Ba(OH)2 + H2↑
Barium combines with several metals, including aluminium, zinc, lead and tin, forming intermetallic phases and alloys.[9]
Isotopes
Naturally occurring barium is a mix of seven stable isotopes, the most abundant being 138Ba (71.7 %). 22 isotopes are known, but most of these are highly radioactive and have half-lives in the several millisecond to several day range. The only notable exceptions are 133Ba which has a half-life of 10.51 years, and 137mBa (2.55 minutes).[10] 133Ba is a standard calibrant for gamma-ray detectors in nuclear physics studies.
Occurrence
The abundance of barium is 0.0425 % in the Earth's crust and 13 µg/L in sea water. It occurs in the minerals barite (as the sulfate) and witherite (as the carbonate).[9] Although witherite deposits were mined from the 17th century till 1969[11] in northern England, for example in the Settlingstones Mine near Newbrough,[12] today nearly all barium is mined as barite.
Large deposits of barite are found in China, Germany, India, Morocco, and in the United States.[13] A rare gem containing barium is known, called benitoite.
Production
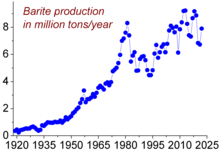
Because barium quickly oxidizes in air, it is difficult to obtain the free metal and it is never found free in nature. The metal is primarily found in, and extracted from, barite. Because barite is so insoluble, it cannot be used directly for the preparation of other barium compounds, or barium metal. Instead, the ore is heated with carbon to reduce it to barium sulfide:[14]
- BaSO4 + 2 C → BaS + 2 CO2
The barium sulfide is then hydrolyzed or treated with acids to form other barium compounds, such as the chloride, nitrate, and carbonate.
Barium is commercially produced through the electrolysis of molten barium chloride (BaCl2):
Barium metal is also obtained by the reduction of barium oxide with finely divided aluminium at temperatures between 1100 and 1200 °C:
- 4 BaO + 2 Al → BaO·Al2O3 + 3 Ba
The barium vapor is cooled and condensed to give the solid metal, which can be cast into rods or extruded into wires. Being a flammable solid, it is packaged under argon in steel containers or plastic bags.[9]
Compounds
Ba2+ is the dominant oxidation state throughout the chemistry of barium. Its properties generally resemble those of other alkaline earth ions such as strontium and calcium. All halides, pseudohalides and chalcogenides are known, usually as colourless solids. The sulfate is famously insoluble. BaO forms a peroxide when heated in air. The oxide is basic and reacts with acids to give salts. Barium reduces oxides, chlorides and sulfides of less active metals. For example:
- Ba + CdO → BaO + Cd
- Ba + ZnCl2 → BaCl2 + Zn
- 3 Ba + Al2S3 → 3 BaS + 2 Al
At elevated temperatures, barium combines with nitrogen and hydrogen to produce the nitride Ba3N2 and hydride BaH2, respectively. When heated with nitrogen and carbon, it forms the cyanide:
- Ba + N2 + 2 C → Ba(CN)2
History
Barium's name originates from Greek βαρύς barys, meaning "heavy", describing the density of some common barium-containing ores. Alchemists in the early Middle Ages knew about some barium minerals. Smooth pebble-like stones of mineral barite found in Bologna, Italy were known as "Bologna stones". Witches and alchemists were attracted to them because after exposure to light they would glow for years.[15]
Carl Scheele identified barite as containing a new element in 1774, but could not isolate barium, only barium oxide. Johan Gottlieb Gahn also isolated barium oxide two years later in similar studies. Oxidized barium was at first called barote, by Guyton de Morveau, a name which was changed by Antoine Lavoisier to baryta. Also in the 18th century, English mineralogist William Withering noted a heavy mineral in the lead mines of cumberland, now known to be Witherite. Barium was first isolated by electrolysis of molten barium salts in 1808, by Sir Humphry Davy in England.[16] Davy, by analogy with calcium named "barium" after baryta, with the "-ium" ending signifying a metallic element.[15] Robert Bunsen and Augustus Matthiessen yielded pure barium by electrolysis of a molten mixture of barium chloride and ammonium chloride.[17][18]
The production of pure oxygen in the Brin process was a large scale application of barium peroxide before electrolysis and fractionally distill liquefied air became the dominant ways to produce oxygen. In this process the barium oxide reacts at 500–600°C with air to form barium peroxide which decomposes at above 700°C by releasing oxygen.[19][20]
- 2 BaO + O2 ⇌ 2 BaO2
Applications


The dominating application of elemental barium is as a scavenger or "getter" removing the last traces of oxygen and other gases in electronic vacuum tubes such as television cathode ray tubes.[9]
An alloy of barium with nickel is commonly used in automobile ignitions.[21]
Applications of barium sulfate
Barium sulfate (the mineral barite, BaSO4) is important to the petroleum industry, for example, as a drilling mud weighting agent in drilling new oil and gas wells.[13] It is also a filler in a variety of products such as rubber. Taking advantage of its opacity to X-rays, the sulfate is used as a radiocontrast agent for X-ray imaging of the digestive system ("barium meals" and "barium enemas").[13] Lithopone, a pigment that contains barium sulfate and zinc sulfide, is a permanent white that has good covering power, and does not darken when exposed to sulfides.[22]
Applications of other barium compounds
Aside from the sulfate, other compounds of barium find only niche applications. Applications are limited by the toxicity of Ba2+ ions (Barium carbonate is a rat poison), which is not a problem for the insoluble BaSO4.
- Barium oxide is used in a coating for the electrodes of fluorescent lamps, which facilitates the release of electrons.
- Barium carbonate is also used in glassmaking. Being a heavy element, barium increases the refractive index and luster of the glass.[13]
- Barium, commonly as barium nitrate, is used to give green colors in fireworks.[23] The species responsible for the brilliant green is barium monochloride; in the absence of a source of chlorine a yellow or "apple" green is produced instead.
- Barium peroxide can be used as a catalyst to start an aluminothermic reaction when welding rail tracks together. It can also be used in green tracer ammunition and as a bleaching agent.[24]
- Barium titanate is a promising electroceramic.
- Barium fluoride is used for optics in infrared applications, since it is transparent from about 0.15 to 12 micrometres.[25]
Precautions
Soluble barium compounds are poisonous. At low doses, barium acts as a muscle stimulant, whereas higher doses affect the nervous system, causing cardiac irregularities, tremors, weakness, anxiety, dyspnea and paralysis. This may be due to its ability to block potassium ion channels which are critical to the proper function of the nervous system.[26] However, individual responses to barium salts vary widely, with some being able to handle barium nitrate casually without problems, and others becoming ill from working with it in small quantities. For example, barium acetate was used by Marie Robards to poison her father in 1993.[27]
Non-toxicity of barium sulfate
Because it is highly insoluble in water as well as stomach acids, barium sulfate can be taken orally. It is eliminated completely from the digestive tract. Unlike other heavy metals, barium does not bioaccumulate.[28][29] However, inhaled dust containing barium compounds can accumulate in the lungs, causing a benign condition called baritosis.[30]
See also
References
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 112. ISBN 978-0-08-037941-8.
- ^ "Standard Atomic Weights: Barium". CIAAW. 1985.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ Stwertka, Albert (2002). A guide to the elements. Oxford University Press US. p. 144. ISBN 0195150279.
- ^ a b c d Robert Kresse, Ulrich Baudis, Paul Jäger, H. Hermann Riechers, Heinz Wagner, Jochen Winkler, Hans Uwe Wolf, "Barium and Barium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2007 Wiley-VCH, Weinheim. doi:10.1002/14356007.a03_325.pub2
- ^ David R. Lide, Norman E. Holden (2005). "Section 11, Table of the Isotopes". CRC Handbook of Chemistry and Physics, 85th Edition. Boca Raton, Florida: CRC Press.
- ^ Industrial minerals. 1969. p. 28.
- ^ "Alston Moor Cumbria, UK". Steetley Minerals.
- ^ a b c d C. R. Hammond (2000). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304814.
- ^ "Toxicological Profile for Barium and Barium Compounds. Agency for Toxic Substances and Disease Registry" (PDF). CDC. 2007.
{{cite web}}: Check date values in:|date=(help) - ^ a b Robert E. Krebs (2006). The history and use of our earth's chemical elements: a reference guide. Greenwood Publishing Group. p. 80. ISBN 0313334382.
- ^ Davy, H. (1808) "Electro-chemical researches on the decomposition of the earths; with observations on the metals obtained from the alkaline earths, and on the amalgam procured from ammonia," Philosophical Transactions of the Royal Society of London, vol. 98, pages 333-370.
- ^ "Masthead". Annalen der Chemie und Pharmacie. 93 (3): fmi–fmi. 1855. doi:10.1002/jlac.18550930301.
- ^ Wagner, Rud.; Neubauer, C.; Deville, H. Sainte-Claire; Sorel; Wagenmann, L.; Techniker; Girard, Aimé (1856). "Notizen". Journal für Praktische Chemie. 67: 490–508. doi:10.1002/prac.18560670194.
- ^ Jensen, William B. (2009). "The Origin of the Brin Process for the Manufacture of Oxygen". Journal of Chemical Education. 86 (11): 1266. Bibcode:2009JChEd..86.1266J. doi:10.1021/ed086p1266.
- ^ Ihde, Aaron John (1984-04-01). The development of modern chemistry. p. 681. ISBN 9780486642352.
- ^ Stellman, Jeanne (1998). Encyclopaedia of Occupational Health and Safety: Chemical, industries and occupations. International Labour Organization. p. 63.8. ISBN 9789221098164.
- ^ Chris J. Jones, John Thornback (2007). Medicinal applications of coordination chemistry. Royal Society of Chemistry. p. 102. ISBN 0854045961.
- ^ Michael S. Russell, Kurt Svrcula (2008). Chemistry of Fireworks. Royal Society of Chemistry. p. 110. ISBN 0854041273.
- ^ Brent, G. F.; Harding, M. D. (1995). "Surfactant coatings for the stabilization of barium peroxide and lead dioxide in pyrotechnic compositions". Propellants Explosives Pyrotechnics. 20 (6): 300. doi:10.1002/prep.19950200604.
- ^ "Crystran Ltd. Optical Component Materials". Retrieved 2010-12-29.
- ^ Patnaik, Pradyot (2003). Handbook of inorganic chemicals. pp. 77–78. ISBN 0070494398.--
- ^ "Boyfriend fight preceded Roanoke mom's slaying" (PDF). Retrieved 2009-06-06.
- ^ "Toxicity Profiles, Ecological Risk Assessment". US EPA. Retrieved 2009-06-06.
- ^ Moore, J. W. (1991). Inorganic Contaminants of Surface Waters, Research and Monitoring Priorities. New York: Springer-Verlag.
- ^ Doig AT (1976). "Baritosis: a benign pneumoconiosis". Thorax. 31 (1): 30–9. doi:10.1136/thx.31.1.30. PMC 470358. PMID 1257935.
{{cite journal}}: Unknown parameter|month=ignored (help)


