Mebendazole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Generic (formerly Vermox) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682315 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | ~2% |
| Metabolism | Hepatic |
| Elimination half-life | 2.5 to 5.5 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.017 |
| Chemical and physical data | |
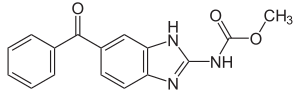
| Formula | C16H13N3O3 |
| Molar mass | 295.293 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 288.5 °C (551.3 °F) |
| |
| |
| (verify) | |
Mebendazole or MBZ is a benzimidazole drug developed by Janssen Pharmaceutica and marketed in the USA as a generic drug only, according to the FDA Orange Book. It is used to treat infestations by worms including pinworms, roundworms, tapeworms, hookworms, and whipworms.
Mebendazole is a WHO Essential Medicine.
Medical use
The drug is a highly effective, broad-spectrum antihelmintic indicated for the treatment of nematode infestations, including roundworm, whipworm, threadworm, and hookworm. It is poorly absorbed and has no systemic effects.[citation needed]
Mechanism
Mebendazole is thought to work by selectively inhibiting the synthesis of microtubules in parasitic worms, and by destroying extant cytoplasmic microtubes in their intestinal cells, thereby blocking the uptake of glucose and other nutrients, resulting in the gradual immobilization and eventual death of the helminths.
permeability of mebendazole
Dosage
Oral dosage for treatment of pinworms is 100 mg taken once. (500mg can also be taken instead) This regimen is repeated two weeks later if the infestation has not cleared up. Oral dosage for treatment of whipworm, common roundworm and hookworm is one 100-mg tablet in the morning for three consecutive days in children below 5yrs of age and below 18 kg weight. In adults it is 100 mg twice a day. [1]
Adverse effects
Mebendazole is relatively free of toxic side effects or adverse reactions, although patients may complain of transient abdominal pain, heart pain, diarrhea, slight headache, fever, dizziness, exanthema, urticaria, and angioedema.
Contraindications
Pregnancy
Mebendazole is contraindicated in pregnant women because it has been shown to be embryotoxic and teratogenic in experimental animals.
Drug interactions
Carbamazepine and phenytoin lower serum levels of mebendazole. Cimetidine does not appreciably raise serum mebendazole (in contrast to the similar drug albendazole), consistent with its poor systemic absorption.[2][3]
Stevens–Johnson syndrome and the more severe toxic epidermal necrolysis can occur when mebendazole is combined with high doses of metronidazole.[4]
Oncologic treatment potential
Several studies show mebendazole exhibits potent antitumor properties. MBZ significantly inhibited cancer cell growth, migration and metastatic formation of adrenocortical carcinoma, both in vitro and in vivo.[5] Treatment of lung cancer cell lines with MBZ caused mitotic arrest, followed by apoptotic cell death with the feature of caspase activation and cytochrome c release.[6] MZ induced a dose- and time-dependent apoptotic response in human lung cancer cell lines,[7] and apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells.[8]
Discontinuation in United States
The last manufacturer of mebendazole in the United States, Teva Pharmaceuticals, announced on October 7, 2011, they have ceased manufacture of this product. As of December, 2011, it is no longer available from any manufacturer in the USA. No reason was given publicly for this discontinuation.[9] Mebendazole formulations can be made by a compounding pharmacy at the request of a doctor.
References
- ^ "Mebendazole". Drugs.com. Retrieved 2011-10-29.
- ^ "Drug Interactions". Medicine chest. Retrieved 2008-05-06.
- ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 3816925 , please use {{cite journal}} with
|pmid= 3816925instead. - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 12604501 , please use {{cite journal}} with
|pmid= 12604501instead. - ^ Martarelli D, Pompei P, Baldi C, Mazzoni G (2008). "Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice". Cancer Chemother. Pharmacol. 61 (5): 809–17. doi:10.1007/s00280-007-0538-0. PMID 17581752.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sasaki J, Ramesh R, Chada S, Gomyo Y, Roth JA, Mukhopadhyay T (2002). "The anthelmintic drug mebendazole induces mitotic arrest and apoptosis by depolymerizing tubulin in non-small cell lung cancer cells". Mol. Cancer Ther. 1 (13): 1201–9. PMID 12479701.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mukhopadhyay T, Sasaki J, Ramesh R, Roth JA (2002). "Mebendazole elicits a potent antitumor effect on human cancer cell lines both in vitro and in vivo". Clin. Cancer Res. 8 (9): 2963–9. PMID 12231542.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Doudican N, Rodriguez A, Osman I, Orlow SJ (2008). "Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells". Mol. Cancer Res. 6 (8): 1308–15. doi:10.1158/1541-7786.MCR-07-2159. PMID 18667591.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Drug Shortages Bulletin 750". American Society of Health-System Pharmacists. Retrieved 12/18/2011.
{{cite web}}: Check date values in:|accessdate=(help)
