Lassa fever
| Lassa fever | |
|---|---|
| Other names | Lassa hemorrhagic fever |
 | |
| Liberian laboratory technicians in personal protective equipment preparing to test Lassa fever samples. | |
| Specialty | Infectious disease |
| Symptoms | Fever, headaches, bleeding[1] |
| Complications | Deafness[1] |
| Usual onset | 1–3 weeks following exposure[1] |
| Causes | Lassa virus[1] |
| Risk factors | Exposure to rodents in West Africa[1] |
| Diagnostic method | Laboratory testing[1] |
| Differential diagnosis | Ebola, malaria, typhoid fever[1] |
| Treatment | Supportive, ribavirin[1] |
| Prognosis | ~1% risk of death[1] |
| Frequency | ~400,000 cases per year[2] |
| Deaths | ~5,000 deaths per year[2] |
Lassa fever, also known as Lassa hemorrhagic fever (LHF), is a type of viral hemorrhagic fever caused by the Lassa virus.[1] Many of those infected by the virus do not develop symptoms.[1] When symptoms occur they typically include fever, weakness, headaches, vomiting, and muscle pains.[1] Less commonly there may be bleeding from the mouth or gastrointestinal tract.[1] The risk of death once infected is about one percent and frequently occurs within two weeks of the onset of symptoms.[1] Among those who survive about a quarter have hearing loss, which improves over time in about half.[1]
The disease is usually initially spread to people via contact with the urine or feces of an infected multimammate rat.[1] Spread can then occur via direct contact between people.[1] Diagnosis based on symptoms is difficult.[1] Confirmation is by laboratory testing to detect the virus's RNA, antibodies for the virus, or the virus itself in cell culture.[1] Other conditions that may present similarly include Ebola, malaria, typhoid fever, and yellow fever.[1] The Lassa virus is a member of the Arenaviridae family of viruses.[1]
There is no vaccine.[3] Prevention requires isolating those who are infected and decreasing contact with the rats.[1] Other efforts to control the spread of disease include having a cat to hunt vermin, and storing food in sealed containers.[1] Treatment is directed at addressing dehydration and improving symptoms.[1] The antiviral medication ribavirin may be useful when given early.[1] These measures improve outcomes.[1]
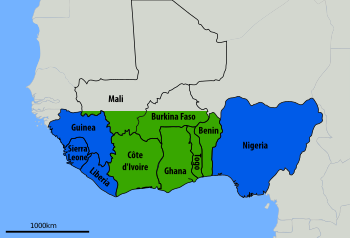
Descriptions of the disease date from the 1950s.[1] The virus was first described in 1969 from a case in the town of Lassa, in Borno State, Nigeria.[1][4] Lassa fever is relatively common in West Africa including the countries of Nigeria, Liberia, Sierra Leone, Guinea, and Ghana.[1][2] There are about 300,000 to 500,000 cases which result in 5,000 deaths a year.[2]
Pathophysiology
Lassa virus is a single-stranded negative sense RNA virus.[5]The transmission of Lassa virus to humans occurs most commonly through aerosols generated from the urine or feces of an infected rodent[6]. Natal multimammate rats shed the virus in urine and droppings and direct contact with these excreta, through touching soiled objects, eating contaminated food, or exposure to open cuts or sores, can lead to infection.[6][7] There have been reports of sexual transmission of Lassa fever but it is rare.[8] High serum virus titres, combined with disseminated replication in tissues and absence of neutralizing antibodies (immuno-compromisation), lead to the development of Lassa fever.[5] However, an intact and active immune response is protective against developing symptoms by mounting the early innate immune response in order to prevent further infection and virus growth, which in turn attenuates humoral and cell-mediated immunity.[6][9][10] Due to limited data on Lassa fever, the immune responses against it and its pathogenesis are poorly understood. As such, it is not well understood how viral infection leads to sepsis-like symptoms, cytokine storms or bacterial co-infection.[11][12]
One possible mechanism involved in Lassa fever pathogenesis could be infection-triggered induction of uncontrolled cytokine expression similar to what is seen in sepsis. Another possibility is that virus-induced immunosuppression may be involved in the pathogenesis of severe Lassa fever disease. Patients infected with Lassa virus produce IgM and IgG antibody isotypes. However since both immunoglobulin classes are detected in viremic patients, most likely the antibodies that are produced early in infection are not neutralizing and remain detectable for many years and can be found in many individuals across West Africa, whereas late antibodies neutralize the virus and are protective.[5][13] Antbodies produced in early infection are not neutralizing because of under-processed glycans form spatially distinct clusters, which shield the proteinous surface of the Lassa virus glycoprotein spike from the humoral immune response,[6][14][15] and inhibited innate immune response.[6][15][16]
The main underlying feature of Lassa fever is that the vascular bed is attacked by the virus, with resultant micro-vascular damage and changes in vascular permeability. Secondary results of capillary leakage and reduced effective circulating blood volume may include increase in sympathetic tone, local tissue acidosis, anoxia and further reduction in tissue blood flow, thus generating the shock syndrome. Overall it is apparent that liver damage occurs in almost all cases of Lassa fever in varying degrees,[5] while vascular damage and hemorrhaging tends to be associated more with the New World arenaviruses in South America.[17]
Pre–renal acute kidney failure, lactic acidaemia, hyperkalaemia and reduced perfusion and oxygenation of vital tissues follows and progress to fatal outcome. The secondary effects of micro-vascular damage include alterations in pulmonary function due to several mechanisms.[18]
Epidemiology
Estimating the true incidence and mortality of Lassa Fever is extremely difficult due to the non-specific clinical presentation; underdeveloped surveillance systems; extensive human migration and perturbation of the physical landscape; and lack of reagents and laboratories for laboratory confirmation.[20]
Nevertheless, Lassa fever frequently infects people in West Africa with approximately 80% being asymptomatic. Studies show up to 300,000 – 500,000 cases annually and about 5,000 deaths. Lassa fever is endemic in parts of West Africa, including Sierra Leone, Liberia, Ghana, Guinea and Nigeria.[21]
There also is evidence of endemicity in neighboring countries; in 2016, two cases were reported in Togo,[22] and 6 confirmed cases in Benin.[23] In the US on 25 May 2015, there was a confirmed case in a US returnee from Liberia.[24] There have been imported cases of Lassa fever in European countries including Sweden,[25] Germany,[26] The Netherlands[27] and United Kingdom,[28] all of which where imported from West Africa.
Outbreak in Nigeria
In Nigeria, From 1 January to 20 May 2018, 1940 suspected cases have been reported from 21 states. Of these, 431 were confirmed positive, 10 are probable, 1495 negative.[27][28] A total of 6489 contacts have been identified in 20 states since January 2019 to March 2019, a total of 2034 suspected cases have been reported from 21 states. Of these, 526 were confirmed positive, 15 probable and 1693 negative (not a case), and 17 health care workers have been affected in six states with four deaths (case fatality rate= 29%).[27]
Signs and symptoms
The incubation period ranges from 6 – 21 days. The onset of the disease is non–specific when symptomatic and usually gradual, starting with fever, general weakness and malaise. After a few days, headache, sore throat, muscle pain, chest pain, nausea, vomiting, diarrhea, cough, and abdominal pain may follow.[29] In severe cases systemic involvement occurs with the following:
- Respiratory: pleural effusion, epistaxis, rales, rhonchi, stridor, cough, wheezing, pharyngitis, and dyspnoea;
- Gastrointestinal: hematemesis, melena, gingival bleeding, dysphagia, hepatitis, and hepatic tenderness;
- Renal: hematuria, dysuria;
- Cardiovascular: Pericarditis, Hypotension, and tachycardia;
- Nervous: encephalitis, cloudy sensorium, seizures, disorientation, and coma, unilateral or bilateral hearing deficit;
- Vascular: Petechial and ecchymotic cuteneous lesions, facial and cervical edema.
Transient hair loss and gait disturbance may occur during recovery.
Death usually occurs within 14 days of onset in fatal cases.[30]
Causes and transmission
Lassa virus is zoonotic,[31] as it spreads specifically from Natal multimammate mice (Mastomys natalensis). It is the most common mouse in equatorial Africa, ubiquitous in human households and eaten as a delicacy in some areas.[32] Infection occurs by exposure to rat excrement directly or indirectly via contaminated food stuffs. Infection can also occur by inhalation of tiny particles (aerosols) of infected materials. There is no epidemiological evidence supporting airborne spread between humans. It is possible to acquire infection through broken skin or mucous membranes that is directly exposed to infectious materials, and through rat bites. In addition, the virus can also be contracted via contaminated medical equipment, such as re–used needles and improper sterilization. The presence of Lassa virus in seminal fluid definitely suggests increased risk of transmission through sexual intercourse but viral dose that the person is exposed to might not be enough to cause clinical infection often enough to contribute significantly to the burden of the clinical disease in these populations.[33]
Diagnosis
Because the symptoms of Lassa fever are so varied and non–specific, clinical diagnosis is often difficult, especially early in the course of the disease. Lassa fever is difficult to distinguish from other febrile disease e.g. malaria, typhoid, influenza, relapsing fever, leptospirosis and other hemorrhagic fevers e.g. yellow fever, dengue fever, Marburg and Ebola virus etc.[34]
The following laboratory tests can be conducted
- Enzyme – linked immunosorbent assays (ELISAs) can be used to detect specific immunoglobulin G (IgG) antibodies or viral antigens in acute serum samples from patients with Lassa fever. (It can be detected even in acute phase)
- Reverse transcriptase polymerase chain reaction (RT–PCR) assay
- Virus cultivation and identification technique (virus isolation by cell culture). However, this requires 3 – 10 days or longer for definitive identification[35][36]
- Blood cultures to differentiate other pathogens (e.g. typhoid) and blood smear to differentiate malaria parasite as the virus can present concomitantly with other diseases
- General biochemical tests such as full blood count, erythrocyte sedimentation rate; hematocrit volume (to exclude anemia); White blood cell count (to exclude lymphopenia); platelet count (to exclude thrombocytopenia), coagulation studies (to exclude coagulopathies) and liver and kidney function tests (serum liver enzymes have been found to be positive clinical markers).[37]
The World Health Organization guidelines for the collection, storage, and handling of specimens for Ebola virus testing should be followed when testing for Lassa virus.[38] Maximal biosafety level (BSL-4) precautions are recommended when handling specimens which may contain infectious Lassa virus.[38] However, the availability of such high-containment laboratories is limited worldwide. If BSL-4 precautions are not available, samples may be handled in a class II or III biosafety cabinets or they may be inactivated to allow safe handling of specimens under BSL-2 precautions.[38][39] The development of appropriate diagnostic assays is further complicated by significant Lassa virus diversity. The high nucleotide and amino acid diversity of Lassa virus isolates sequenced across West Africa can result in false-negative results if the primer/probe or antibody pairs do not bind to the target sufficiently. For example, a commonly used reverse transcriptase PCR (RT-PCR) assay[38] was redesigned when false negatives were identified due to primer-template mismatches.[38]
Currently, two National Laboratories in Nigeria are supporting the laboratory confirmation PCR tests. All the samples are also tested for Ebola, dengue and yellow fever (which have so far tested negative).[40]
Management
Supportive (symptomatic) management includes bed rest; close observation and monitoring; serial laboratory tests; analgesics (e.g. acetaminophen); tepid sponging and antipyretic drugs to reduce fever; anti-emetic drugs (e.g. metoclopramide and promethazine); prompt fluid and electrolyte replacement; diuretics (e.g. furosemide) for fluid retention; oxygen therapy; blood and/or platelet transfusion; and management of other complications.
In terms of specific management, early aggressive treatment using ribavirin appears to be the most effective treatment if given early on in the course of clinical illness.[41][42] Intravenous interferon may also be given alongside ribavirin.
Pharmacology of ribavirin
The generic drug ribavirin is a broad–spectrum antiviral nucleoside (guanosine). Its international brand names include Copegus, Ibavyr, Moderiba, Virazole, Virazide, Rebetol, Ribasphere, RibaTab and Riboflax, among many others.[43]
Mode of action
Although the mechanism of ribavirin remains unclear, ribavirin appears to be a non–specific antiviral agent with most of its efficacy due to incorporation of ribavirin into the viral genome. When cells are exposed to ribavirin, there is reduction in intracellular guanosine triphosphate (a requirement for translation, transcription and replication in viruses). Therefore ribavirin significantly inhibits viral replication and translation by inhibiting DNA and RNA synthesis.[44]
Contraindications
Documented or known hypersensitivity, compromised renal function, or renal failure (creatinine clearance <30 ml/min), pregnancy, hemoglobinopathies (e.g. Thelassemia major, sickle cell anemia (with hemoglobin level less than 8g/dl) etc.) are (relative) contraindications to ribavirin.
Drug interactions
Ribavirin inhibits the phosphorylation of zidovudine and ostavudin.[45]
Adverse effects
Adverse effects include:
- Hemolytic anemia: This may occur 1 – 2 weeks after initiation of therapy. It is recommended that hematocrit count is obtained pre–treatment and at week 2 and week 4 of therapy or more frequently if clinically indicated.
- Fatal and no-fatal myocardial infarction have been reported in patients with anemia caused by ribavirin. Patients should be assessed for underlying cardiac disease before initiation of therapy. Patients with pre–existing cardiac disease should have electrocardiography done before treatment and should be appropriately monitored during therapy.
- Hypersensitivity: e.g. uticaria, angioedema, bronchoconstriction and anaphylaxis
- Bone marrow suppression (Pancytopenia)
- Unusual tiredness and weakness
- insomnia, depression, irritability and suicidal behavior have been reported with oral administration
- Ocular problems
- Mild hepatic and renal impairment.[46]
Ribavirin in pregnancy
Ribavirin may cause birth defects and/or death of exposed fetuses. Ribavirin has demonstrated significant teratogenic and/or embryocidal effects in all animal species in which adequate studies have been conducted. These effects occurred at doses as low as one twentieth of the recommended human doses of ribavirin.[47]
Ribavirin therapy should not be started unless a report of pregnancy negative test has been obtained immediately prior to planned initiation of therapy.[a] Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients (as ribavirin can be excreted via sperm). Patients should be instructed to use at least two forms of effective contraception during treatment and for 6 months after treatment has been stopped. Pregnancy testing should occur monthly during ribavirin therapy and for six months after therapy has stopped.[48][49]
Post Exposure Prophylaxis (PEP)
Patients who come in contact with infected patients or equipment (i.e. via broken skin, mucous membrane or needle stick injuries) approximately within 2 days of exposure, are given 800 mg of ribavirin daily or 400 mg twice daily for 10 days. This was the proposal of Vito et al. in 2010, following their experimental research in Sierra Leone's Lassa ward on only 25 people who were exposed to the virus, all being negative after the prophylaxis. But there is no substantial evidence to support the effectiveness of immediate initiation of PEP.[50]
However the CDC recommends placing high-risk exposed individuals under medical surveillance for 21 days and treating presumptively with ribavirin if clinical evidence of viral hemorrhagic fever develops.[51]
Prevention and control

Prevention of Lassa fever relies on prompting good “community hygiene” to discourage rodents from entering homes. Effective measures include storing grain and other foodstuffs in rodent–proof containers, disposing of garbage far from the home, maintaining clean households and keeping cats. Because Mastomys rats are so abundant in endemic areas, it is not possible to completely eliminate them from the environment but it is possible to control contact with them. Family members should avoid contact with blood and other bodily fluid while caring for sick persons and should observe safe burial practices.[52]
In health care settings, staffs should always apply standard infection prevention and control precautions when caring for patients, regardless of their presumed diagnosis. These include basic hand hygiene, respiratory hygiene, use of personal protective equipment (to block splashes or other contact with infected materials), handling of laboratory samples with caution, and safe injection practices.[53][54]
Proper isolation of suspected and confirmed cases of Lassa fever, good quarantine protocols, health education and rigorous contact tracing should be employed by the government and health care agencies. Drugs, equipment and appropriate expertise should also be readily available to control the spread in time.[55]
Treatment
All persons suspected of Lassa fever infection should be admitted to isolation facilities and their body fluids and excreta properly disposed of.
Early and aggressive treatment using ribavirin was pioneered by Joe McCormick in 1979. After extensive testing, early administration was determined to be critical to success. Additionally, ribavirin is almost twice as effective when given intravenously as when taken by mouth.[56] Ribavirin is a prodrug which appears to interfere with viral replication by inhibiting RNA-dependent nucleic acid synthesis, although the precise mechanism of action is disputed.[57] The drug is relatively inexpensive, but the cost of the drug is still very high for many of those in West African states. Fluid replacement, blood transfusion, and fighting hypotension are usually required. Intravenous interferon therapy has also been used.
When Lassa fever infects pregnant women late in their third trimester, inducing delivery is necessary for the mother to have a good chance of survival.[58] This is because the virus has an affinity for the placenta and other highly vascular tissues. The fetus has only a one in ten chance of survival no matter what course of action is taken; hence, the focus is always on saving the life of the mother.[59] Following delivery, women should receive the same treatment as other Lassa fever patients.
Work on a vaccine is continuing, with multiple approaches showing positive results in animal trials.[60]
Prognosis
About 15–20% of hospitalized Lassa fever patients will die from the illness. The overall mortality rate is estimated to be 1%, but during epidemics, mortality can climb as high as 50%. The mortality rate is greater than 80% when it occurs in pregnant women during their third trimester; fetal death also occurs in nearly all those cases. Abortion decreases the risk of death to the mother.[61] Some survivors experience lasting effects of the disease,[62] and can include partial or complete deafness.[1]
Because of treatment with ribavirin, fatality rates are continuing to decline.[63][64]
Research
The Lassa virus is one of several viruses identified by WHO as a likely cause of a future epidemic. They therefore list it for urgent research and development to develop new diagnostic tests, vaccines, and medicines.[65][66]
In 2007, SIGA Technologies, studied a medication in guinea pig with Lassa fever.[67]
See also
References
A wikidata item for the academic article needs to be provided (search Wikidata). See template documentation for details.)
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad "Lassa fever". WHO. March 2016. Archived from the original on 1 November 2016. Retrieved 2 November 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d Ogbu O, Ajuluchukwu E, Uneke CJ (2007). "Lassa fever in West African sub-region: an overview". Journal of Vector Borne Diseases. 44 (1): 1–11. PMID 17378212.
Lassa fever is endemic in West Africa.
- ^ Yun, N. E.; Walker, D. H. (2012). "Pathogenesis of Lassa Fever". Viruses. 4 (12): 2031–2048. doi:10.3390/v4102031. PMC 3497040. PMID 23202452.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Frame JD, Baldwin JM, Gocke DJ, Troup JM (1 July 1970). "Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings". Am. J. Trop. Med. Hyg. 19 (4): 670–6. doi:10.4269/ajtmh.1970.19.670. PMID 4246571. Archived from the original on 14 March 2008.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d Yun, Nadezhda E.; Walker, David H. (9 October 2012). "Pathogenesis of Lassa Fever". Viruses. 4 (10): 2031–2048. doi:10.3390/v4102031. ISSN 1999-4915. PMC 3497040. PMID 23202452.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c d e Hallam, Hoai J.; Hallam, Steven; Rodriguez, Sergio E.; Barrett, Alan D. T.; Beasley, David W. C.; Chua, Arlene; Ksiazek, Thomas G.; Milligan, Gregg N.; Sathiyamoorthy, Vaseeharan (20 March 2018). "Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development". NPJ Vaccines. 3. doi:10.1038/s41541-018-0049-5. ISSN 2059-0105. PMC 5861057. PMID 29581897.
- ^ "Transmission of Lassa Fever". www.cdc.gov. 6 March 2019. Retrieved 9 April 2019.
- ^ Oshin, Babafemi A. (9 April 2019). "Rat eating, sexual transmission and the burden of Lassa fever disease".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Zapata, Juan Carlos; Medina-Moreno, Sandra; Guzmán-Cardozo, Camila; Salvato, Maria S. (28 October 2018). "Improving the Breadth of the Host's Immune Response to Lassa Virus". Pathogens. 7 (4). doi:10.3390/pathogens7040084. ISSN 2076-0817. PMC 6313495. PMID 30373278.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Brosh-Nissimov, Tal (30 April 2016). "Lassa fever: another threat from West Africa". Disaster and Military Medicine. 2. doi:10.1186/s40696-016-0018-3. ISSN 2054-314X. PMC 5330145. PMID 28265442.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Lin, Gu-Lung; McGinley, Joseph P.; Drysdale, Simon B.; Pollard, Andrew J. (27 September 2018). "Epidemiology and Immune Pathogenesis of Viral Sepsis". Frontiers in Immunology. 9. doi:10.3389/fimmu.2018.02147. ISSN 1664-3224. PMC 6170629. PMID 30319615.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ USA US9193705B2, Cunningham J; Lee K & Ren T et al., "Small molecule inhibitors of ebola and lassa fever viruses and methods of use"
- ^ "Lassa". Viral Hemorrhagic Fever Consortium. Retrieved 9 April 2019.
- ^ Crispin, Max; Bowden, Thomas A.; Strecker, Thomas; Huiskonen, Juha T.; Moser, Felipe; Li, Sai; Seabright, Gemma E.; Allen, Joel D.; Raghwani, Jayna (10 July 2018). "Structure of the Lassa virus glycan shield provides a model for immunological resistance". Proceedings of the National Academy of Sciences. 115 (28): 7320–7325. doi:10.1073/pnas.1803990115. ISSN 0027-8424. PMID 29941589.
- ^ a b Robinson, James E.; Hastie, Kathryn M.; Cross, Robert W.; Yenni, Rachael E.; Elliott, Deborah H.; Rouelle, Julie A.; Kannadka, Chandrika B.; Smira, Ashley A.; Garry, Courtney E. (10 May 2016). "Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits". Nature Communications. 7. doi:10.1038/ncomms11544. ISSN 2041-1723. PMC 4866400. PMID 27161536.
- ^ McCormick, J. B.; Brown, B.; Hutwagner, L.; Fisher-Hoch, S. P. (1 August 2000). "Effective Vaccine for Lassa Fever". Journal of Virology. 74 (15): 6777–6783. doi:10.1128/JVI.74.15.6777-6783.2000. ISSN 0022-538X. PMID 10888616.
- ^ Brisse, Morgan E.; Ly, Hinh (13 March 2019). "Hemorrhagic Fever-Causing Arenaviruses: Lethal Pathogens and Potent Immune Suppressors". Frontiers in Immunology. 10. doi:10.3389/fimmu.2019.00372. ISSN 1664-3224. PMC 6424867. PMID 30918506.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Monath, T. P.; Casals, J. (1975). "Diagnosis of Lassa fever and the isolation and management of patients". Bulletin of the World Health Organization. 52 (4–6): 707–715. ISSN 0042-9686. PMC 2366641. PMID 1085225.
{{cite journal}}: CS1 maint: PMC format (link) - ^ "Outbreak Distribution Map of Lassa Fever". www.cdc.gov. CDC. 4 March 2019. Retrieved 27 April 2019.
- ^ Grant, Donald S.; Khan, Humarr; Schieffelin, John; Bausch, Daniel G. (2014). Emerging Infectious Diseases. Elsevier. pp. 37–59. doi:10.1016/b978-0-12-416975-3.00004-2. ISBN 9780124169753.
- ^ Behrens, Ron; Houlihan, Catherine (12 July 2017). "Lassa fever". BMJ. 358: j2986. doi:10.1136/bmj.j2986. ISSN 1756-1833. PMID 28701331.
- ^ "Lassa Fever – Togo". WHO. Retrieved 11 January 2019.
- ^ "Lassa Fever – Benin". WHO. Retrieved 11 January 2019.
- ^ "Lassa Fever – United States of America". WHO. Retrieved 11 January 2019.
- ^ "Lassa fever – Sweden". WHO. Retrieved 11 January 2019.
- ^ "Lassa Fever – Germany". WHO. Retrieved 11 January 2019.
- ^ "2000 - Imported case of Lassa fever in The Netherlands - Update". WHO. Retrieved 11 January 2019.
- ^ "Imported case of Lassa fever in United Kingdom". WHO. Retrieved 11 January 2019.
- ^ Centers for Disease Control and Prevention, "Lassa Fever" Archived 23 September 2016 at the Wayback Machine
- ^ Greenky, David; Knust, Barbara; Dziuban, Eric J. (1 May 2018). "What Pediatricians Should Know About Lassa Virus". JAMA pediatrics. 172 (5): 407–408. doi:10.1001/jamapediatrics.2017.5223. ISSN 2168-6203. PMC 5970952. PMID 29507948.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Kafetzopoulou, L. E.; Pullan, S. T.; Lemey, P.; Suchard, M. A.; Ehichioya, D. U.; Pahlmann, M.; Thielebein, A.; Hinzmann, J.; Oestereich, L. (4 January 2019). "Metagenomic sequencing at the epicenter of the Nigeria 2018 Lassa fever outbreak". Science. 363 (6422): 74–77. doi:10.1126/science.aau9343. ISSN 0036-8075.
- ^ Hussainia, Nafiu; Abdulhamid, Abdurrahman (1 January 2018). "Effects of quarantine on transmission dynamics of Lassa fever". Bayero Journal of Pure and Applied Sciences. 11 (1): 397–407–407. ISSN 2006-6996.
- ^ Oshin, Babafemi A. (27 April 2019). "Rat eating, sexual transmission and the burden of Lassa fever disease".
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Lassa fever". www.who.int. Retrieved 11 January 2019.
- ^ Raabe, Vanessa; Koehler, Jeffrey (2017-6). Kraft, Colleen Suzanne (ed.). "Laboratory Diagnosis of Lassa Fever". Journal of Clinical Microbiology. 55 (6): 1629–1637. doi:10.1128/JCM.00170-17. ISSN 0095-1137. PMC 5442519. PMID 28404674.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Panning, Marcus; Emmerich, Petra; Ölschläger, Stephan; Bojenko, Sergiusz; Koivogui, Lamine; Marx, Arthur; Lugala, Peter Clement; Günther, Stephan; Bausch, Daniel G. (2010-6). "Laboratory Diagnosis of Lassa Fever, Liberia". Emerging Infectious Diseases. 16 (6): 1041–1043. doi:10.3201/eid1606.100040. ISSN 1080-6040. PMC 3086251. PMID 20507774.
{{cite journal}}: Check date values in:|date=(help)CS1 maint: PMC format (link) - ^ Salvato, Maria S.; Lukashevich, Igor S.; Medina-Moreno, Sandra; Zapata, Juan Carlos (2018). "Diagnostics for Lassa Fever: Detecting Host Antibody Responses". Methods in Molecular Biology (Clifton, N.J.). 1604: 79–88. doi:10.1007/978-1-4939-6981-4_5. ISSN 1940-6029. PMID 28986826.
- ^ a b c d e Raabe, Vanessa; Koehler, Jeffrey (06 2017). "Laboratory Diagnosis of Lassa Fever". Journal of Clinical Microbiology. 55 (6): 1629–1637. doi:10.1128/JCM.00170-17. ISSN 1098-660X. PMC 5442519. PMID 28404674.
{{cite journal}}: Check date values in:|date=(help) - ^ Asogun, Danny A.; Adomeh, Donatus I.; Ehimuan, Jacqueline; Odia, Ikponmwonsa; Hass, Meike; Gabriel, Martin; Ölschläger, Stephan; Becker-Ziaja, Beate; Folarin, Onikepe (27 September 2012). "Molecular Diagnostics for Lassa Fever at Irrua Specialist Teaching Hospital, Nigeria: Lessons Learnt from Two Years of Laboratory Operation". PLoS Neglected Tropical Diseases. 6 (9). doi:10.1371/journal.pntd.0001839. ISSN 1935-2727. PMC 3459880. PMID 23029594.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Olalekan, Adebimpe Wasiu (2 November 2016). "Pre-epidemic preparedness and the control of Lassa fever in Southern Nigeria". Research Journal of Health Sciences. 4 (3): 243. doi:10.4314/rejhs.v4i3.7. ISSN 2467-8252.
- ^ Raabe, Vanessa N.; Kann, Gerrit; Ribner, Bruce S.; Morales, Andres; Varkey, Jay B.; Mehta, Aneesh K.; Lyon, G. Marshall; Vanairsdale, Sharon; Faber, Kelly (1 September 2017). "Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever". Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 65 (5): 855–859. doi:10.1093/cid/cix406. ISSN 1058-4838. PMC 5682919. PMID 29017278.
- ^ "Lassa fever". www.who.int. Retrieved 11 January 2019.
- ^ "Ribavirin". www.drugbank.ca. Retrieved 25 May 2019.
- ^ Tyring, Stephen K. (Stephen Keith) (2005). Antiviral agents, vaccines, and immunotherapies. New York: Marcel Dekker. ISBN 9780824754082. OCLC 58604581.
- ^ "Rebetol (Ribavirin): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved 25 May 2019.
- ^ "Common Side Effects of Rebetol (Ribavirin) Drug Center". RxList. Retrieved 25 May 2019.
- ^ "Common Side Effects of Rebetol (Ribavirin) Drug Center". RxList. Retrieved 25 May 2019.
- ^ "Rebetol (Ribavirin): Side Effects, Interactions, Warning, Dosage & Uses". RxList. Retrieved 25 May 2019.
- ^ "Rebetol, Ribasphere (ribavirin) dosing, indications, interactions, adverse effects, and more". reference.medscape.com. Retrieved 25 May 2019.
- ^ "Ribavirin for Lassa Fever Postexposure Prophylaxis" (PDF). wwwnc.cdc.gov. Retrieved 16 April 2017.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Al, C. M. Hadi et. "Ribavirin for Lassa Fever Postexposure Prophylaxis - Volume 16, Number 12—December 2010 - Emerging Infectious Diseases journal - CDC". doi:10.3201/eid1612.100994.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Lassa fever". www.who.int. Retrieved 25 May 2019.
- ^ "SterlingHealthMCS - LASSA FEVER". www.sterlinghealthmcs.com. Retrieved 25 May 2019.
- ^ Ogbu, O.; Ajuluchukwu, E.; Uneke, C. J. (2007-3). "Lassa fever in West African sub-region: an overview". Journal of Vector Borne Diseases. 44 (1): 1–11. ISSN 0972-9062. PMID 17378212.
{{cite journal}}: Check date values in:|date=(help) - ^ I., Donaldson, Ross (2009). The Lassa ward : one man's fight against one of the world's deadliest diseases (1st ed ed.). New York: St. Martin's Press. ISBN 0312377002. OCLC 262885308.
{{cite book}}:|edition=has extra text (help)CS1 maint: multiple names: authors list (link) - ^ Fisher-Hoch SP, McCormick JB (2004). "Lassa fever vaccine". Expert Review of Vaccines. 3 (2): 189–97. doi:10.1586/14760584.3.4.S189. PMID 15056044.
- ^ Crotty S, Cameron C, Andino R (2002). "Ribavirin's antiviral mechanism of action: lethal mutagenesis?". J. Mol. Med. 80 (2): 86–95. doi:10.1007/s00109-001-0308-0. PMID 11907645.
- ^ Price ME, Fisher-Hoch SP, Craven RB, McCormick JB (September 1988). "A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy". BMJ. 297 (6648): 584–7. doi:10.1136/bmj.297.6648.584. PMC 1834487. PMID 3139220.
- ^ Samuel, Daso. "Lassa fever... What you need to know" (PDF). Archived from the original (PDF) on 25 June 2017. Retrieved 1 February 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "WHO Target Product Profiles for Lassa virus Vaccine" (PDF). World Health Organization. April 2017. Retrieved 11 September 2017.
{{cite web}}: Cite has empty unknown parameter:|dead-url=(help) - ^ Centers for Disease Control and Prevention, "Lassa Fever, Signs and Symptoms" Archived 9 July 2017 at the Wayback Machine
- ^ Emond, R. T.; Bannister, B.; Lloyd, G.; Southee, T. J.; Bowen, E. T. (1982). "A case of Lassa fever: Clinical and virological findings". British Medical Journal (Clinical Research Ed.). 285 (6347): 1001–1002. doi:10.1136/bmj.285.6347.1001. PMC 1500383. PMID 6812716.
- ^ "Lassa fever". World Health Organization. Retrieved 11 September 2017.
- ^ McCormick, J. B.; King, I. J.; Webb, P. A.; Scribner, C. L.; Craven, R. B.; Johnson, K. M.; Elliott, L. H.; Belmont-Williams, R. (2 January 1986). "Lassa fever. Effective therapy with ribavirin". The New England Journal of Medicine. 314 (1): 20–26. doi:10.1056/NEJM198601023140104. ISSN 0028-4793. PMID 3940312.
- ^ Kieny, Marie-Paule. "After Ebola, a Blueprint Emerges to Jump-Start R&D". Scientific American Blog Network. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "LIST OF PATHOGENS". World Health Organization. Archived from the original on 20 December 2016. Retrieved 13 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "SIGA Technologies says passes first hurdle with lassa fever..." Reuters. 15 May 2007. Retrieved 1 May 2019.
Further reading
- Echioya, Deborah U.; Hass, Meike; Olshlager, Stephan; Becker-Ziaja, Beate; Chukwu, Christian O. Onyebuchi; Coker, Jide; Nasidi, Abdulsalam; Ogugua, Osi-Ogdu; Gunther, Stephan; Omilabu, Sunday A. (2010). "Lassa Fever, Nigeria, 2005-2008". Emerging Infectious Diseases. 6. 16 (6): 1040–41. doi:10.3201/eid1606.100080. PMC 3086228. PMID 20507773.
- Branco, Luis M.; Grove, Jessica N.; Boisen, Matt L.; Shaffer, Jeffrey G.; Goba, Augustine; Fullah, Mohammed; Momoh, Mambu; Grant, Donald S.; Garry, Robert F. (4 October 2011). "Emerging Trends in Lassa Fever: Redefining the Role of Immunoglobulin M and Inflammation in Diagnosing Acute Infection". Virology Journal. 8: 478. doi:10.1186/1743-422x-8-478. PMC 3223505. PMID 22023795.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
Cite error: There are <ref group=lower-alpha> tags or {{efn}} templates on this page, but the references will not show without a {{reflist|group=lower-alpha}} template or {{notelist}} template (see the help page).
