Gene therapy
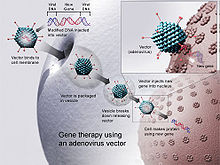
Gene therapy (also called human gene transfer) is a medical field which focuses on the utilization of the therapeutic delivery of nucleic acid into a patient's cells as a drug to treat disease.[1][2] The first attempt at modifying human DNA was performed in 1980 by Martin Cline, but the first successful nuclear gene transfer in humans, approved by the National Institutes of Health, was performed in May 1989.[3] The first therapeutic use of gene transfer as well as the first direct insertion of human DNA into the nuclear genome was performed by French Anderson in a trial starting in September 1990. It is thought to be able to cure many genetic disorders or treat them over time.
Between 1989 and December 2018, over 2,900 clinical trials were conducted, with more than half of them in phase I.[4] As of 2017, Spark Therapeutics' Luxturna (RPE65 mutation-induced blindness) and Novartis' Kymriah (Chimeric antigen receptor T cell therapy) are the FDA's first approved gene therapies to enter the market. Since that time, drugs such as Novartis' Zolgensma and Alnylam's Patisiran have also received FDA approval, in addition to other companies' gene therapy drugs. Most of these approaches utilize adeno-associated viruses (AAVs) and lentiviruses for performing gene insertions, in vivo and ex vivo, respectively. ASO / siRNA approaches such as those conducted by Alnylam and Ionis Pharmaceuticals require non-viral delivery systems, and utilize alternative mechanisms for trafficking to liver cells by way of GalNAc transporters.
The introduction of CRISPR gene editing has opened new doors for its application and utilization in gene therapy. Solutions to medical hurdles, such as the eradication of latent human immunodeficiency virus (HIV) reservoirs, may soon become a tangible reality.[5][6]
Not all medical procedures that introduce alterations to a patient's genetic makeup can be considered gene therapy. Bone marrow transplantation and organ transplants in general have been found to introduce foreign DNA into patients.[7] Gene therapy is defined by the precision of the procedure and the intention of direct therapeutic effect.
Background
Gene therapy was conceptualized in 1972, by authors who urged caution before commencing human gene therapy studies.
The first attempt, an unsuccessful one, at gene therapy (as well as the first case of medical transfer of foreign genes into humans not counting organ transplantation) was performed by Martin Cline on 10 July 1980.[8][9] Cline claimed that one of the genes in his patients was active six months later, though he never published this data or had it verified[10] and even if he is correct, it's unlikely it produced any significant beneficial effects treating beta-thalassemia.
After extensive research on animals throughout the 1980s and a 1989 bacterial gene tagging trial on humans, the first gene therapy widely accepted as a success was demonstrated in a trial that started on 14 September 1990, when Ashi DeSilva was treated for ADA-SCID.[11]
The first somatic treatment that produced a permanent genetic change was initiated in 1993. The goal was to cure malignant brain tumors by using recombinant DNA to transfer a gene making the tumor cells sensitive to a drug that in turn would cause the tumor cells to die.[12]
Gene therapy is a way to fix a genetic problem at its source. The polymers are either translated into proteins, interfere with target gene expression, or possibly correct genetic mutations.
The most common form uses DNA that encodes a functional, therapeutic gene to replace a mutated gene. The polymer molecule is packaged within a "vector", which carries the molecule inside cells.
Early clinical failures led to dismissals of gene therapy. Clinical successes since 2006 regained researchers' attention, although as of 2014[update], it was still largely an experimental technique.[13] These include treatment of retinal diseases Leber's congenital amaurosis[14][15][16][17] and choroideremia,[18] X-linked SCID,[19] ADA-SCID,[20][21] adrenoleukodystrophy,[22] chronic lymphocytic leukemia (CLL),[23] acute lymphocytic leukemia (ALL),[24] multiple myeloma,[25] haemophilia,[21] and Parkinson's disease.[26] Between 2013 and April 2014, US companies invested over $600 million in the field.[27]
The first commercial gene therapy, Gendicine, was approved in China in 2003 for the treatment of certain cancers.[28] In 2011 Neovasculgen was registered in Russia as the first-in-class gene-therapy drug for treatment of peripheral artery disease, including critical limb ischemia.[29] In 2012 Glybera, a treatment for a rare inherited disorder, lipoprotein lipase deficiency became the first treatment to be approved for clinical use in either Europe or the United States after its endorsement by the European Commission.[13][30]
Following early advances in genetic engineering of bacteria, cells, and small animals, scientists started considering how to apply it to medicine. Two main approaches were considered – replacing or disrupting defective genes.[31] Scientists focused on diseases caused by single-gene defects, such as cystic fibrosis, haemophilia, muscular dystrophy, thalassemia, and sickle cell anemia. Glybera treats one such disease, caused by a defect in lipoprotein lipase.[30]
DNA must be administered, reach the damaged cells, enter the cell and either express or disrupt a protein.[32] Multiple delivery techniques have been explored. The initial approach incorporated DNA into an engineered virus to deliver the DNA into a chromosome.[33][34] Naked DNA approaches have also been explored, especially in the context of vaccine development.[35]
Generally, efforts focused on administering a gene that causes a needed protein to be expressed. More recently, increased understanding of nuclease function has led to more direct DNA editing, using techniques such as zinc finger nucleases and CRISPR. The vector incorporates genes into chromosomes. The expressed nucleases then knock out and replace genes in the chromosome. As of 2014[update] these approaches involve removing cells from patients, editing a chromosome and returning the transformed cells to patients.[36]
Gene editing is a potential approach to alter the human genome to treat genetic diseases,[37] viral diseases,[38] and cancer.[citation needed] As of 2016[update] these approaches were still years from being medicine.[39][40]

Cell types
Gene therapy may be classified into two types:
Somatic
In somatic cell gene therapy (SCGT), the therapeutic genes are transferred into any cell other than a gamete, germ cell, gametocyte, or undifferentiated stem cell. Any such modifications affect the individual patient only, and are not inherited by offspring. Somatic gene therapy represents mainstream basic and clinical research, in which therapeutic DNA (either integrated in the genome or as an external episome or plasmid) is used to treat disease.
Over 600 clinical trials utilizing SCGT are underway[when?] in the US. Most focus on severe genetic disorders, including immunodeficiencies, haemophilia, thalassaemia, and cystic fibrosis. Such single gene disorders are good candidates for somatic cell therapy. The complete correction of a genetic disorder or the replacement of multiple genes is not yet possible. Only a few of the trials are in the advanced stages.[41] [needs update]
Germline
In germline gene therapy (GGT), germ cells (sperm or egg cells) are modified by the introduction of functional genes into their genomes. Modifying a germ cell causes all the organism's cells to contain the modified gene. The change is therefore heritable and passed on to later generations. Australia, Canada, Germany, Israel, Switzerland, and the Netherlands[42] prohibit GGT for application in human beings, for technical and ethical reasons, including insufficient knowledge about possible risks to future generations[42] and higher risks versus SCGT.[43] The US has no federal controls specifically addressing human genetic modification (beyond FDA regulations for therapies in general).[42][44][45][46]
Vectors
The delivery of DNA into cells can be accomplished by multiple methods. The two major classes are recombinant viruses (sometimes called biological nanoparticles or viral vectors) and naked DNA or DNA complexes (non-viral methods).
Viruses
In order to replicate, viruses introduce their genetic material into the host cell, tricking the host's cellular machinery into using it as blueprints for viral proteins. Retroviruses go a stage further by having their genetic material copied into the genome of the host cell. Scientists exploit this by substituting a virus's genetic material with therapeutic DNA. (The term 'DNA' may be an oversimplification, as some viruses contain RNA, and gene therapy could take this form as well.) A number of viruses have been used for human gene therapy, including retroviruses, adenoviruses, herpes simplex, vaccinia, and adeno-associated virus.[4] Like the genetic material (DNA or RNA) in viruses, therapeutic DNA can be designed to simply serve as a temporary blueprint that is degraded naturally or (at least theoretically) to enter the host's genome, becoming a permanent part of the host's DNA in infected cells.
Non-viral
Non-viral methods present certain advantages over viral methods, such as large scale production and low host immunogenicity. However, non-viral methods initially produced lower levels of transfection and gene expression, and thus lower therapeutic efficacy. Newer technologies offer promise of solving these problems, with the advent of increased cell-specific targeting and subcellular trafficking control.
Methods for non-viral gene therapy include the injection of naked DNA, electroporation, the gene gun, sonoporation, magnetofection, the use of oligonucleotides, lipoplexes, dendrimers, and inorganic nanoparticles.
More recent approaches, such as those performed by companies such as Ligandal, offer the possibility of creating cell-specific targeting technologies for a variety of gene therapy modalities, including RNA, DNA and gene editing tools such as CRISPR. Other companies, such as Arbutus Biopharma and Arcturus Therapeutics, offer non-viral, non-cell-targeted approaches that mainly exhibit liver trophism. In more recent years, startups such as Sixfold Bio, GenEdit, and Spotlight Therapeutics have begun to solve the non-viral gene delivery problem. Non-viral techniques offer the possibility of repeat dosing and greater tailorability of genetic payloads, which in the future will be more likely to take over viral-based delivery systems.
Companies such as Editas Medicine, Intellia Therapeutics, CRISPR Therapeutics, Casebia, Cellectis, Precision Biosciences, bluebird bio, and Sangamo have developed non-viral gene editing techniques, however frequently still use viruses for delivering gene insertion material following genomic cleavage by guided nucleases. These companies focus on gene editing, and still face major delivery hurdles.
Moderna Therapeutics and CureVac focus on delivery of mRNA payloads, which are necessarily non-viral delivery problems.
Alnylam, Dicerna Pharmaceuticals, and Ionis Pharmaceuticals focus on delivery of siRNA (antisense oligonucleotides) for gene suppression, which also necessitate non-viral delivery systems.
In academic contexts, a number of laboratories are working on delivery of PEGylated particles, which form serum protein coronas and chiefly exhibit LDL receptor mediated uptake in cells in vivo.[47]
Hurdles
Some of the unsolved problems include:
- Short-lived nature – Before gene therapy can become a permanent cure for a condition, the therapeutic DNA introduced into target cells must remain functional and the cells containing the therapeutic DNA must be stable. Problems with integrating therapeutic DNA into the genome and the rapidly dividing nature of many cells prevent it from achieving long-term benefits. Patients require multiple treatments.
- Immune response – Any time a foreign object is introduced into human tissues, the immune system is stimulated to attack the invader. Stimulating the immune system in a way that reduces gene therapy effectiveness is possible. The immune system's enhanced response to viruses that it has seen before reduces the effectiveness to repeated treatments.
- Problems with viral vectors – Viral vectors carry the risks of toxicity, inflammatory responses, and gene control and targeting issues.
- Multigene disorders – Some commonly occurring disorders, such as heart disease, high blood pressure, Alzheimer's disease, arthritis, and diabetes, are affected by variations in multiple genes, which complicate gene therapy.
- Some therapies may breach the Weismann barrier (between soma and germ-line) protecting the testes, potentially modifying the germline, falling afoul of regulations in countries that prohibit the latter practice.[48]
- Insertional mutagenesis – If the DNA is integrated in a sensitive spot in the genome, for example in a tumor suppressor gene, the therapy could induce a tumor. This has occurred in clinical trials for X-linked severe combined immunodeficiency (X-SCID) patients, in which hematopoietic stem cells were transduced with a corrective transgene using a retrovirus, and this led to the development of T cell leukemia in 3 of 20 patients.[49][50] One possible solution is to add a functional tumor suppressor gene to the DNA to be integrated. This may be problematic since the longer the DNA is, the harder it is to integrate into cell genomes.[51] CRISPR technology allows researchers to make much more precise genome changes at exact locations.[52]
- Cost – Alipogene tiparvovec or Glybera, for example, at a cost of $1.6 million per patient, was reported in 2013 to be the world's most expensive drug.[53][54]
Deaths
Three patients' deaths have been reported in gene therapy trials, putting the field under close scrutiny. The first was that of Jesse Gelsinger, who died in 1999 because of immune rejection response.[55] One X-SCID patient died of leukemia in 2003.[11] An 18-year-old male died of systemic inflammatory response syndrome following adenovirus gene therapy in 2003.[56] In 2007, a rheumatoid arthritis patient died from an infection; the subsequent investigation concluded that the death was not related to gene therapy.[57] However it is always important to remember that although deaths are rare they can still occur and it is very possible that certain types of gene therapy can cause certain cancers.
History
This section may be too long and excessively detailed. (November 2018) |
1970s and earlier
In 1972 Friedmann and Roblin authored a paper in Science titled "Gene therapy for human genetic disease?"[58] Rogers (1970) was cited for proposing that exogenous good DNA be used to replace the defective DNA in those who suffer from genetic defects.[59]
1980s
In 1984 a retrovirus vector system was designed that could efficiently insert foreign genes into mammalian chromosomes.[60]
1990s
The first approved gene therapy clinical research in the US took place on 14 September 1990, at the National Institutes of Health (NIH), under the direction of William French Anderson.[61] Four-year-old Ashanti DeSilva received treatment for a genetic defect that left her with ADA-SCID, a severe immune system deficiency. The defective gene of the patient's blood cells was replaced by the functional variant. Ashanti's immune system was partially restored by the therapy. Production of the missing enzyme was temporarily stimulated, but the new cells with functional genes were not generated. She led a normal life only with the regular injections performed every two months. The effects were successful, but temporary.[62]
Cancer gene therapy was introduced in 1992/93 (Trojan et al. 1993).[63] The treatment of glioblastoma multiforme, the malignant brain tumor whose outcome is always fatal, was done using a vector expressing antisense IGF-I RNA (clinical trial approved by NIH protocol no.1602 24 November 1993,[64] and by the FDA in 1994). This therapy also represents the beginning of cancer immunogene therapy, a treatment which proves to be effective due to the anti-tumor mechanism of IGF-I antisense, which is related to strong immune and apoptotic phenomena.
In 1992 Claudio Bordignon, working at the Vita-Salute San Raffaele University, performed the first gene therapy procedure using hematopoietic stem cells as vectors to deliver genes intended to correct hereditary diseases.[65] In 2002 this work led to the publication of the first successful gene therapy treatment for adenosine deaminase deficiency (ADA-SCID). The success of a multi-center trial for treating children with SCID (severe combined immune deficiency or "bubble boy" disease) from 2000 and 2002, was questioned when two of the ten children treated at the trial's Paris center developed a leukemia-like condition. Clinical trials were halted temporarily in 2002, but resumed after regulatory review of the protocol in the US, the United Kingdom, France, Italy, and Germany.[66]
In 1993 Andrew Gobea was born with SCID following prenatal genetic screening. Blood was removed from his mother's placenta and umbilical cord immediately after birth, to acquire stem cells. The allele that codes for adenosine deaminase (ADA) was obtained and inserted into a retrovirus. Retroviruses and stem cells were mixed, after which the viruses inserted the gene into the stem cell chromosomes. Stem cells containing the working ADA gene were injected into Andrew's blood. Injections of the ADA enzyme were also given weekly. For four years T cells (white blood cells), produced by stem cells, made ADA enzymes using the ADA gene. After four years more treatment was needed.[67]
Jesse Gelsinger's death in 1999 impeded gene therapy research in the US.[68][69] As a result, the FDA suspended several clinical trials pending the reevaluation of ethical and procedural practices.[70]
2000s
The modified cancer gene therapy strategy of antisense IGF-I RNA (NIH n˚ 1602)[64] using antisense / triple helix anti-IGF-I approach was registered in 2002 by Wiley gene therapy clinical trial - n˚ 635 and 636. The approach has shown promising results in the treatment of six different malignant tumors: glioblastoma, cancers of liver, colon, prostate, uterus, and ovary (Collaborative NATO Science Programme on Gene Therapy USA, France, Poland n˚ LST 980517 conducted by J. Trojan) (Trojan et al., 2012). This anti-gene antisense/triple helix therapy has proven to be efficient, due to the mechanism stopping simultaneously IGF-I expression on translation and transcription levels, strengthening anti-tumor immune and apoptotic phenomena.
2002
Sickle-cell disease can be treated in mice.[71] The mice – which have essentially the same defect that causes human cases – used a viral vector to induce production of fetal hemoglobin (HbF), which normally ceases to be produced shortly after birth. In humans, the use of hydroxyurea to stimulate the production of HbF temporarily alleviates sickle cell symptoms. The researchers demonstrated this treatment to be a more permanent means to increase therapeutic HbF production.[72]
A new gene therapy approach repaired errors in messenger RNA derived from defective genes. This technique has the potential to treat thalassaemia, cystic fibrosis and some cancers.[73]
Researchers created liposomes 25 nanometers across that can carry therapeutic DNA through pores in the nuclear membrane.[74]
2003
In 2003 a research team inserted genes into the brain for the first time. They used liposomes coated in a polymer called polyethylene glycol, which unlike viral vectors, are small enough to cross the blood–brain barrier.[75]
Short pieces of double-stranded RNA (short, interfering RNAs or siRNAs) are used by cells to degrade RNA of a particular sequence. If a siRNA is designed to match the RNA copied from a faulty gene, then the abnormal protein product of that gene will not be produced.[76]
Gendicine is a cancer gene therapy that delivers the tumor suppressor gene p53 using an engineered adenovirus. In 2003, it was approved in China for the treatment of head and neck squamous cell carcinoma.[28]
2006
In March researchers announced the successful use of gene therapy to treat two adult patients for X-linked chronic granulomatous disease, a disease which affects myeloid cells and damages the immune system. The study is the first to show that gene therapy can treat the myeloid system.[77]
In May a team reported a way to prevent the immune system from rejecting a newly delivered gene.[78] Similar to organ transplantation, gene therapy has been plagued by this problem. The immune system normally recognizes the new gene as foreign and rejects the cells carrying it. The research utilized a newly uncovered network of genes regulated by molecules known as microRNAs. This natural function selectively obscured their therapeutic gene in immune system cells and protected it from discovery. Mice infected with the gene containing an immune-cell microRNA target sequence did not reject the gene.
In August scientists successfully treated metastatic melanoma in two patients using killer T cells genetically retargeted to attack the cancer cells.[79]
In November researchers reported on the use of VRX496, a gene-based immunotherapy for the treatment of HIV that uses a lentiviral vector to deliver an antisense gene against the HIV envelope. In a phase I clinical trial, five subjects with chronic HIV infection who had failed to respond to at least two antiretroviral regimens were treated. A single intravenous infusion of autologous CD4 T cells genetically modified with VRX496 was well tolerated. All patients had stable or decreased viral load; four of the five patients had stable or increased CD4 T cell counts. All five patients had stable or increased immune response to HIV antigens and other pathogens. This was the first evaluation of a lentiviral vector administered in a US human clinical trial.[80][81]
2007
In May researchers announced the first gene therapy trial for inherited retinal disease. The first operation was carried out on a 23-year-old British male, Robert Johnson, in early 2007.[82]
2008
Leber's congenital amaurosis is an inherited blinding disease caused by mutations in the RPE65 gene. The results of a small clinical trial in children were published in April.[14] Delivery of recombinant adeno-associated virus (AAV) carrying RPE65 yielded positive results. In May two more groups reported positive results in independent clinical trials using gene therapy to treat the condition. In all three clinical trials, patients recovered functional vision without apparent side-effects.[14][15][16][17]
2009
In September researchers were able to give trichromatic vision to squirrel monkeys.[83] In November 2009, researchers halted a fatal genetic disorder called adrenoleukodystrophy in two children using a lentivirus vector to deliver a functioning version of ABCD1, the gene that is mutated in the disorder.[84]
2010s
2010
An April paper reported that gene therapy addressed achromatopsia (color blindness) in dogs by targeting cone photoreceptors. Cone function and day vision were restored for at least 33 months in two young specimens. The therapy was less efficient for older dogs.[85]
In September it was announced that an 18-year-old male patient in France with beta-thalassemia major had been successfully treated.[86] Beta-thalassemia major is an inherited blood disease in which beta haemoglobin is missing and patients are dependent on regular lifelong blood transfusions.[87] The technique used a lentiviral vector to transduce the human ß-globin gene into purified blood and marrow cells obtained from the patient in June 2007.[88] The patient's haemoglobin levels were stable at 9 to 10 g/dL. About a third of the hemoglobin contained the form introduced by the viral vector and blood transfusions were not needed.[88][89] Further clinical trials were planned.[90] Bone marrow transplants are the only cure for thalassemia, but 75% of patients do not find a matching donor.[89]
Cancer immunogene therapy using modified antigene, antisense/triple helix approach was introduced in South America in 2010/11 in La Sabana University, Bogota (Ethical Committee 14 December 2010, no P-004-10). Considering the ethical aspect of gene diagnostic and gene therapy targeting IGF-I, the IGF-I expressing tumors i.e. lung and epidermis cancers were treated (Trojan et al. 2016).[91][92]
2011
In 2007 and 2008, a man (Timothy Ray Brown) was cured of HIV by repeated hematopoietic stem cell transplantation (see also allogeneic stem cell transplantation, allogeneic bone marrow transplantation, allotransplantation) with double-delta-32 mutation which disables the CCR5 receptor. This cure was accepted by the medical community in 2011.[93] It required complete ablation of existing bone marrow, which is very debilitating.
In August two of three subjects of a pilot study were confirmed to have been cured from chronic lymphocytic leukemia (CLL). The therapy used genetically modified T cells to attack cells that expressed the CD19 protein to fight the disease.[23] In 2013, the researchers announced that 26 of 59 patients had achieved complete remission and the original patient had remained tumor-free.[94]
Human HGF plasmid DNA therapy of cardiomyocytes is being examined as a potential treatment for coronary artery disease as well as treatment for the damage that occurs to the heart after myocardial infarction.[95][96]
In 2011 Neovasculgen was registered in Russia as the first-in-class gene-therapy drug for treatment of peripheral artery disease, including critical limb ischemia; it delivers the gene encoding for VEGF.[97][29] Neovasculogen is a plasmid encoding the CMV promoter and the 165 amino acid form of VEGF.[98][99]
2012
The FDA approved Phase 1 clinical trials on thalassemia major patients in the US for 10 participants in July.[100] The study was expected to continue until 2015.[90]
In July 2012, the European Medicines Agency recommended approval of a gene therapy treatment for the first time in either Europe or the United States. The treatment used Alipogene tiparvovec (Glybera) to compensate for lipoprotein lipase deficiency, which can cause severe pancreatitis.[101] The recommendation was endorsed by the European Commission in November 2012[13][30][102][103] and commercial rollout began in late 2014.[104] Alipogene tiparvovec was expected to cost around $1.6 million per treatment in 2012,[105] revised to $1 million in 2015,[106] making it the most expensive medicine in the world at the time.[107] As of 2016[update], only the patients treated in clinical trials and a patient who paid the full price for treatment have received the drug.[108]
In December 2012, it was reported that 10 of 13 patients with multiple myeloma were in remission "or very close to it" three months after being injected with a treatment involving genetically engineered T cells to target proteins NY-ESO-1 and LAGE-1, which exist only on cancerous myeloma cells.[25]
2013
In March researchers reported that three of five adult subjects who had acute lymphocytic leukemia (ALL) had been in remission for five months to two years after being treated with genetically modified T cells which attacked cells with CD19 genes on their surface, i.e. all B-cells, cancerous or not. The researchers believed that the patients' immune systems would make normal T-cells and B-cells after a couple of months. They were also given bone marrow. One patient relapsed and died and one died of a blood clot unrelated to the disease.[24]
Following encouraging Phase 1 trials, in April, researchers announced they were starting Phase 2 clinical trials (called CUPID2 and SERCA-LVAD) on 250 patients[109] at several hospitals to combat heart disease. The therapy was designed to increase the levels of SERCA2, a protein in heart muscles, improving muscle function.[110] The FDA granted this a Breakthrough Therapy Designation to accelerate the trial and approval process.[111] In 2016 it was reported that no improvement was found from the CUPID 2 trial.[112]
In July researchers reported promising results for six children with two severe hereditary diseases had been treated with a partially deactivated lentivirus to replace a faulty gene and after 7–32 months. Three of the children had metachromatic leukodystrophy, which causes children to lose cognitive and motor skills.[113] The other children had Wiskott-Aldrich syndrome, which leaves them to open to infection, autoimmune diseases, and cancer.[114] Follow up trials with gene therapy on another six children with Wiskott-Aldrich syndrome were also reported as promising.[115][116]
In October researchers reported that two children born with adenosine deaminase severe combined immunodeficiency disease (ADA-SCID) had been treated with genetically engineered stem cells 18 months previously and that their immune systems were showing signs of full recovery. Another three children were making progress.[21] In 2014 a further 18 children with ADA-SCID were cured by gene therapy.[117] ADA-SCID children have no functioning immune system and are sometimes known as "bubble children."[21]
Also in October researchers reported that they had treated six hemophilia sufferers in early 2011 using an adeno-associated virus. Over two years later all six were producing clotting factor.[21][118]
2014
In January researchers reported that six choroideremia patients had been treated with adeno-associated virus with a copy of REP1. Over a six-month to two-year period all had improved their sight.[119][120] By 2016, 32 patients had been treated with positive results and researchers were hopeful the treatment would be long-lasting.[18] Choroideremia is an inherited genetic eye disease with no approved treatment, leading to loss of sight.
In March researchers reported that 12 HIV patients had been treated since 2009 in a trial with a genetically engineered virus with a rare mutation (CCR5 deficiency) known to protect against HIV with promising results.[121][122]
Clinical trials of gene therapy for sickle cell disease were started in 2014.[123][124]
In February LentiGlobin BB305, a gene therapy treatment undergoing clinical trials for treatment of beta thalassemia gained FDA "breakthrough" status after several patients were able to forgo the frequent blood transfusions usually required to treat the disease.[125]
In March researchers delivered a recombinant gene encoding a broadly neutralizing antibody into monkeys infected with simian HIV; the monkeys' cells produced the antibody, which cleared them of HIV. The technique is named immunoprophylaxis by gene transfer (IGT). Animal tests for antibodies to ebola, malaria, influenza, and hepatitis were underway.[126][127]
In March, scientists, including an inventor of CRISPR, Jennifer Doudna, urged a worldwide moratorium on germline gene therapy, writing "scientists should avoid even attempting, in lax jurisdictions, germline genome modification for clinical application in humans" until the full implications "are discussed among scientific and governmental organizations".[128][129][130][131]
In October, researchers announced that they had treated a baby girl, Layla Richards, with an experimental treatment using donor T-cells genetically engineered using TALEN to attack cancer cells. One year after the treatment she was still free of her cancer (a highly aggressive form of acute lymphoblastic leukaemia [ALL]).[132] Children with highly aggressive ALL normally have a very poor prognosis and Layla's disease had been regarded as terminal before the treatment.[133]
In December, scientists of major world academies called for a moratorium on inheritable human genome edits, including those related to CRISPR-Cas9 technologies[134] but that basic research including embryo gene editing should continue.[135]
2016
In April the Committee for Medicinal Products for Human Use of the European Medicines Agency endorsed a gene therapy treatment called Strimvelis[136][137] and the European Commission approved it in June.[138] This treats children born with adenosine deaminase deficiency and who have no functioning immune system. This was the second gene therapy treatment to be approved in Europe.[139]
In October, Chinese scientists reported they had started a trial to genetically modify T-cells from 10 adult patients with lung cancer and reinject the modified T-cells back into their bodies to attack the cancer cells. The T-cells had the PD-1 protein (which stops or slows the immune response) removed using CRISPR-Cas9.[140][141]
A 2016 Cochrane systematic review looking at data from four trials on topical cystic fibrosis transmembrane conductance regulator (CFTR) gene therapy does not support its clinical use as a mist inhaled into the lungs to treat cystic fibrosis patients with lung infections. One of the four trials did find weak evidence that liposome-based CFTR gene transfer therapy may lead to a small respiratory improvement for people with CF. This weak evidence is not enough to make a clinical recommendation for routine CFTR gene therapy.[142]
2017
In February Kite Pharma announced results from a clinical trial of CAR-T cells in around a hundred people with advanced Non-Hodgkin lymphoma.[143]
In March, French scientists reported on clinical research of gene therapy to treat sickle-cell disease.[144]
In August, the FDA approved tisagenlecleucel for acute lymphoblastic leukemia.[145] Tisagenlecleucel is an adoptive cell transfer therapy for B-cell acute lymphoblastic leukemia; T cells from a person with cancer are removed, genetically engineered to make a specific T-cell receptor (a chimeric T cell receptor, or "CAR-T") that reacts to the cancer, and are administered back to the person. The T cells are engineered to target a protein called CD19 that is common on B cells. This is the first form of gene therapy to be approved in the United States. In October, a similar therapy called axicabtagene ciloleucel was approved for non-Hodgkin lymphoma.[146]
In December the results of using an adeno-associated virus with blood clotting factor VIII to treat nine haemophilia A patients were published. Six of the seven patients on the high dose regime increased the level of the blood clotting VIII to normal levels. The low and medium dose regimes had no effect on the patient's blood clotting levels.[147][148]
In December, the FDA approved Luxturna, the first in vivo gene therapy, for the treatment of blindness due to Leber's congenital amaurosis.[149] The price of this treatment was 850,000 US dollars for both eyes.[150][151]
2018
A need was identified for high quality randomised controlled trials assessing the risks and benefits involved with gene therapy for people with sickle cell disease.[152]
2019
In February, medical scientists working with Sangamo Therapeutics, headquartered in Richmond, California, announced the first ever "in body" human gene editing therapy to permanently alter DNA - in a patient with Hunter Syndrome.[153] Clinical trials by Sangamo involving gene editing using Zinc Finger Nuclease (ZFN) are ongoing.[154]
In May, the FDA approved Zolgensma for treating spinal muscular atrophy in children under 2 years. The list price of Zolgensma was set at $2.125 million per dose, making it the most expensive drug ever.[155]
In June, the EMA approved Zynteglo for treating beta thalassemia for patients 12 years or older.[156]
In July, Allergan and Editas Medicine announced phase 1/2 clinical trial of AGN-151587 for the treatment of Leber congenital amaurosis 10.[157] It will be the world's first in vivo study of a CRISPR-based human gene editing therapy, where the editing takes place inside the human body.[158]
Speculative uses
Speculated uses for gene therapy include:
Gene doping
Athletes might adopt gene therapy technologies to improve their performance.[159] Gene doping is not known to occur, but multiple gene therapies may have such effects. Kayser et al. argue that gene doping could level the playing field if all athletes receive equal access. Critics claim that any therapeutic intervention for non-therapeutic/enhancement purposes compromises the ethical foundations of medicine and sports.[160]
Human genetic engineering
Genetic engineering could be used to cure diseases, but also to change physical appearance, metabolism, and even improve physical capabilities and mental faculties such as memory and intelligence. Ethical claims about germline engineering include beliefs that every fetus has a right to remain genetically unmodified, that parents hold the right to genetically modify their offspring, and that every child has the right to be born free of preventable diseases.[161][162][163] For parents, genetic engineering could be seen as another child enhancement technique to add to diet, exercise, education, training, cosmetics, and plastic surgery.[164][165] Another theorist claims that moral concerns limit but do not prohibit germline engineering.[166]
A recent issue of the journal Bioethics was devoted to moral issues surrounding germline genetic engineering in people.[167]
Possible regulatory schemes include a complete ban, provision to everyone, or professional self-regulation. The American Medical Association’s Council on Ethical and Judicial Affairs stated that "genetic interventions to enhance traits should be considered permissible only in severely restricted situations: (1) clear and meaningful benefits to the fetus or child; (2) no trade-off with other characteristics or traits; and (3) equal access to the genetic technology, irrespective of income or other socioeconomic characteristics."[168]
As early in the history of biotechnology as 1990, there have been scientists opposed to attempts to modify the human germline using these new tools,[169] and such concerns have continued as technology progressed.[170][171] With the advent of new techniques like CRISPR, in March 2015 a group of scientists urged a worldwide moratorium on clinical use of gene editing technologies to edit the human genome in a way that can be inherited.[128][129][130][131] In April 2015, researchers sparked controversy when they reported results of basic research to edit the DNA of non-viable human embryos using CRISPR.[172][173] A committee of the American National Academy of Sciences and National Academy of Medicine gave qualified support to human genome editing in 2017[174][175] once answers have been found to safety and efficiency problems "but only for serious conditions under stringent oversight."[176]
Regulations
Regulations covering genetic modification are part of general guidelines about human-involved biomedical research. There are no international treaties which are legally binding in this area, but there are recommendations for national laws from various bodies.
The Helsinki Declaration (Ethical Principles for Medical Research Involving Human Subjects) was amended by the World Medical Association's General Assembly in 2008. This document provides principles physicians and researchers must consider when involving humans as research subjects. The Statement on Gene Therapy Research initiated by the Human Genome Organization (HUGO) in 2001 provides a legal baseline for all countries. HUGO's document emphasizes human freedom and adherence to human rights, and offers recommendations for somatic gene therapy, including the importance of recognizing public concerns about such research.[177]
United States
No federal legislation lays out protocols or restrictions about human genetic engineering. This subject is governed by overlapping regulations from local and federal agencies, including the Department of Health and Human Services, the FDA and NIH's Recombinant DNA Advisory Committee. Researchers seeking federal funds for an investigational new drug application, (commonly the case for somatic human genetic engineering,) must obey international and federal guidelines for the protection of human subjects.[178]
NIH serves as the main gene therapy regulator for federally funded research. Privately funded research is advised to follow these regulations. NIH provides funding for research that develops or enhances genetic engineering techniques and to evaluate the ethics and quality in current research. The NIH maintains a mandatory registry of human genetic engineering research protocols that includes all federally funded projects.
An NIH advisory committee published a set of guidelines on gene manipulation.[179] The guidelines discuss lab safety as well as human test subjects and various experimental types that involve genetic changes. Several sections specifically pertain to human genetic engineering, including Section III-C-1. This section describes required review processes and other aspects when seeking approval to begin clinical research involving genetic transfer into a human patient.[180] The protocol for a gene therapy clinical trial must be approved by the NIH's Recombinant DNA Advisory Committee prior to any clinical trial beginning; this is different from any other kind of clinical trial.[179]
As with other kinds of drugs, the FDA regulates the quality and safety of gene therapy products and supervises how these products are used clinically. Therapeutic alteration of the human genome falls under the same regulatory requirements as any other medical treatment. Research involving human subjects, such as clinical trials, must be reviewed and approved by the FDA and an Institutional Review Board.[181][182]
Popular culture
Gene therapy is the basis for the plotline of the film I Am Legend[183] and the TV show Will Gene Therapy Change the Human Race?.[184] In 1994, gene therapy was a plot element in "The Erlenmeyer Flask", the first-season finale of The X-Files; it is also used in Stargate as a means of allowing humans to use Ancient technology.[185][186]
See also
References
- ^ Ermak, Gennady (2015). Emerging Medical Technologies. World Scientific. ISBN 978-981-4675-81-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Kaji, Eugene H. (7 February 2001). "Gene and Stem Cell Therapies". JAMA. 285 (5): 545–550. doi:10.1001/jama.285.5.545. ISSN 0098-7484. PMID 11176856.
- ^ Rosenberg SA, Aebersold P, Cornetta K, Kasid A, Morgan RA, Moen R, et al. (August 1990). "Gene transfer into humans—immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction". The New England Journal of Medicine. 323 (9): 570–578. doi:10.1056/NEJM199008303230904. PMID 2381442.
- ^ a b Gene Therapy Clinical Trials Worldwide Database. The Journal of Gene Medicine. Wiley (June 2016)
- ^ Gupta, Rajat M.; Musunuru, Kiran (1 October 2014). "Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9". The Journal of Clinical Investigation. 124 (10): 4154–4161. doi:10.1172/JCI72992. ISSN 0021-9738. PMC 4191047. PMID 25271723.
- ^ Sanches-da-Silva, Gabriela De Nardi; Medeiros, Luiza Fonseca Sales; Lima, Fabio Mitsuo (21 August 2019). "The Potential Use of the CRISPR-Cas System for HIV-1 Gene Therapy". International Journal of Genomics. 2019: 8458263. doi:10.1155/2019/8458263. ISSN 2314-436X. PMC 6721108. PMID 31531340.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Zimmer, Carl (16 September 2013). "DNA Double Take". The New York Times.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ U.S. Congress, Office of Technology Assessment (December 1984). Human gene therapy – A background paper. DIANE Publishing. ISBN 978-1-4289-2371-3.
- ^ Sun M (October 1982). "Martin Cline loses appeal on NIH grant". Science. 218 (4567): 37. Bibcode:1982Sci...218...37S. doi:10.1126/science.7123214. PMID 7123214.
- ^ Lowenstein, P.R. (2008). "Gene Therapy for Neurological Disorders: New Therapies or Human Experimentation?". In Burley, Justine; Harris, John (eds.). A Companion to Genethics. John Wiley & Sons. ISBN 978-0-470-75637-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Sheridan C (February 2011). "Gene therapy finds its niche". Nature Biotechnology. 29 (2): 121–128. doi:10.1038/nbt.1769. PMID 21301435.
- ^ Oldfield, Edward; Ram, Zvi; Culver, Kenneth; Blaese, Michael; DeVroom, Hetty; Anderson, French (4 February 1993). "Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir". Human Gene Therapy. 4 (1): 39–69. doi:10.1089/hum.1993.4.1-39. PMID 8384892.
- ^ a b c Richards, Sabrina (6 November 2012). "Gene Therapy Arrives in Europe". The Scientist.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, et al. (May 2008). "Safety and efficacy of gene transfer for Leber's congenital amaurosis". The New England Journal of Medicine. 358 (21): 2240–2248. doi:10.1056/NEJMoa0802315. PMC 2829748. PMID 18441370.
- ^ a b Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, et al. (March 2010). "Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration". Molecular Therapy. 18 (3): 643–650. doi:10.1038/mt.2009.277. PMC 2839440. PMID 19953081.
- ^ a b Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Roman AJ, Byrne BJ, Jacobson SG (August 2009). "Vision 1 year after gene therapy for Leber's congenital amaurosis". The New England Journal of Medicine. 361 (7): 725–727. doi:10.1056/NEJMc0903652. PMC 2847775. PMID 19675341.
- ^ a b Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. (May 2008). "Effect of gene therapy on visual function in Leber's congenital amaurosis". The New England Journal of Medicine. 358 (21): 2231–2239. doi:10.1056/NEJMoa0802268. PMID 18441371.
- ^ a b Ghosh, Pallab (28 April 2016). "Gene therapy reverses sight loss and is long-lasting". BBC News, Science & Environment. Retrieved 29 April 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M (June 2010). "20 years of gene therapy for SCID". Nature Immunology. 11 (6): 457–460. doi:10.1038/ni0610-457. PMID 20485269.
- ^ Ferrua F, Brigida I, Aiuti A (December 2010). "Update on gene therapy for adenosine deaminase-deficient severe combined immunodeficiency". Current Opinion in Allergy and Clinical Immunology. 10 (6): 551–556. doi:10.1097/ACI.0b013e32833fea85. PMID 20966749.
- ^ a b c d e Geddes, Linda (30 October 2013) 'Bubble kid' success puts gene therapy back on track. The New Scientist. Retrieved 2 November 2013
- ^ Cartier N, Aubourg P (July 2010). "Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy". Brain Pathology. 20 (4): 857–862. doi:10.1111/j.1750-3639.2010.00394.x. PMID 20626747.
- ^ a b Ledford, H. (2011). "Cell therapy fights leukaemia". Nature. doi:10.1038/news.2011.472.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Coghlan A (26 March 2013). "Gene therapy cures leukaemia in eight days". The New Scientist. Retrieved 15 April 2013.
- ^ a b Coghlan, Andy (11 December 2013). "Souped-up immune cells force leukaemia into remission". New Scientist. Retrieved 15 April 2013.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, et al. (April 2011). "AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial". The Lancet. Neurology. 10 (4): 309–319. doi:10.1016/S1474-4422(11)70039-4. PMID 21419704.
- ^ Herper, Matthew (26 March 2014). "Gene Therapy's Big Comeback". Forbes. Retrieved 28 April 2014.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Pearson S, Jia H, Kandachi K (January 2004). "China approves first gene therapy". Nature Biotechnology. 22 (1): 3–4. doi:10.1038/nbt0104-3. PMID 14704685.
- ^ a b "Gene Therapy for PAD Approved". 6 December 2011. Retrieved 5 August 2015.
- ^ a b c Gallagher, James. (2 November 2012) BBC News – Gene therapy: Glybera approved by European Commission. BBC. Retrieved 15 December 2012.
- ^ U.S. National Library of Medicine, Genomics Home Reference. What is gene therapy? Archived 6 April 2016 at the Wayback Machine
- ^ U.S. National Library of Medicine, Genomics Home Reference. How does gene therapy work?
- ^ Pezzoli D, Chiesa R, De Nardo L, Candiani G (September 2012). "We still have a long way to go to effectively deliver genes!". Journal of Applied Biomaterials & Functional Materials. 10 (2): 82–91. doi:10.5301/JABFM.2012.9707. PMID 23015375.
- ^ Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M (January 2013). "Viral vectors: a look back and ahead on gene transfer technology". The New Microbiologica. 36 (1): 1–22. PMID 23435812.
- ^ Gothelf A, Gehl J (November 2012). "What you always needed to know about electroporation based DNA vaccines". Human Vaccines & Immunotherapeutics. 8 (11): 1694–1702. doi:10.4161/hv.22062. PMC 3601144. PMID 23111168.
- ^ Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD (September 2010). "Genome editing with engineered zinc finger nucleases". Nature Reviews Genetics. 11 (9): 636–646. doi:10.1038/nrg2842. PMID 20717154.
- ^ Bak, Rasmus O.; Gomez-Ospina, Natalia; Porteus, Matthew H. (August 2018). "Gene Editing on Center Stage". Trends in Genetics. 34 (8): 600–611. doi:10.1016/j.tig.2018.05.004. ISSN 0168-9525. PMID 29908711.
- ^ Stone D, Niyonzima N, Jerome KR (September 2016). "Genome editing and the next generation of antiviral therapy". Human Genetics. 135 (9): 1071–1082. doi:10.1007/s00439-016-1686-2. PMC 5002242. PMID 27272125.
- ^ Ledford, Heidi (12 October 2016). "CRISPR deployed to combat sickle-cell anaemia". Nature. Retrieved 13 October 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Grens, Kerry (13 October 2016). "CRISPR Corrects Sickle Cell-Causing Gene In Human Cells". The Scientist. Retrieved 10 October 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Mavilio F, Ferrari G (July 2008). "Genetic modification of somatic stem cells. The progress, problems and prospects of a new therapeutic technology". EMBO Reports. 9 Suppl 1: S64–69. doi:10.1038/embor.2008.81. PMC 3327547. PMID 18578029.
- ^ a b c "International Law". The Genetics and Public Policy Center, Johns Hopkins University Berman Institute of Bioethics. 2010. Archived from the original on 2 September 2014.
- ^ Strachnan T, Read AP (2004). Human Molecular Genetics (3rd ed.). Garland Publishing. p. 616. ISBN 978-0-8153-4184-0.
- ^ Hanna K (2006). "Germline Gene Transfer". National Human Genome Research Institute.
- ^ "Human Cloning and Genetic Modification". Association of Reproductive Health Officials. 2013. Archived from the original on 18 June 2013.
- ^ "Gene Therapy". ama-assn.org. 4 April 2014. Retrieved 22 March 2015.
- ^ Bertrand, Nicolas; Grenier, Philippe; Mahmoudi, Morteza; Lima, Eliana M.; Appel, Eric A.; Dormont, Flavio; Lim, Jong-Min; Karnik, Rohit; Langer, Robert; Farokhzad, Omid C. (3 October 2017). "Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics". Nature Communications. 8 (1): 1–8. doi:10.1038/s41467-017-00600-w. ISSN 2041-1723.
- ^ Korthof G. "The implications of Steele's soma-to-germline feedback for human gene therapy".
- ^ Woods NB, Bottero V, Schmidt M, von Kalle C, Verma IM (April 2006). "Gene therapy: therapeutic gene causing lymphoma". Nature. 440 (7088): 1123. Bibcode:2006Natur.440.1123W. doi:10.1038/4401123a. PMID 16641981.
- ^ Thrasher AJ, Gaspar HB, Baum C, Modlich U, Schambach A, Candotti F, et al. (September 2006). "Gene therapy: X-SCID transgene leukaemogenicity". Nature. 443 (7109): E5–6, discussion E6–7. Bibcode:2006Natur.443E...5T. doi:10.1038/nature05219. PMID 16988659.
- ^ Bak, Rasmus O.; Porteus, Matthew H. (18 July 2017). "CRISPR-Mediated Integration of Large Gene Cassettes Using AAV Donor Vectors". Cell Reports. 20 (3): 750–756. doi:10.1016/j.celrep.2017.06.064. ISSN 2211-1247. PMC 5568673. PMID 28723575.
- ^ Rojahn, Susan Young (11 February 2014). "Genome Surgery". MIT Technology Review. Retrieved 17 February 2014.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gene therapy needs a hero to live up to the hype. New Scientist (31 October 2013) . Retrieved 2 November 2012
- ^ Crasto, Anthony Melvin (7 July 2013). "Glybera – The Most Expensive Drug in the world & First Approved Gene Therapy in the West". All About Drugs. Retrieved 2 November 2013.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ ORNL.gov. ORNL.gov. Retrieved 15 December 2012.
- ^ Raper, Steven E.; Chirmule, Narendra; Lee, Frank S.; Wivel, Nelson A.; Bagg, Adam; Gao, Guang-ping; Wilson, James M.; Batshaw, Mark L. (September 2003). "Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer". Molecular Genetics and Metabolism. 80 (1–2): 148–158. doi:10.1016/j.ymgme.2003.08.016. ISSN 1096-7192. PMID 14567964.
- ^ Frank, Karen M.; Hogarth, D. Kyle; Miller, Jonathan L.; Mandal, Saptarshi; Mease, Philip J.; Samulski, R. Jude; Weisgerber, Glen A.; Hart, John (July 2009). "Investigation of the cause of death in a gene-therapy trial". The New England Journal of Medicine. 361 (2): 161–169. doi:10.1056/NEJMoa0801066. PMID 19587341.
- ^ Friedmann T, Roblin R (March 1972). "Gene therapy for human genetic disease?". Science. 175 (4025): 949–955. Bibcode:1972Sci...175..949F. doi:10.1126/science.175.4025.949. PMID 5061866.
- ^ Rogers S, New Scientist 1970, p. 194
- ^ Cepko CL, Roberts BE, Mulligan RC (July 1984). "Construction and applications of a highly transmissible murine retrovirus shuttle vector". Cell. 37 (3): 1053–1062. doi:10.1016/0092-8674(84)90440-9. PMID 6331674.
- ^ "The first gene therapy". Life Sciences Foundation. 21 June 2011. Archived from the original on 28 November 2012. Retrieved 7 January 2014.
- ^ Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, et al. (October 1995). "T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years". Science. 270 (5235): 475–480. Bibcode:1995Sci...270..475B. doi:10.1126/science.270.5235.475. PMID 7570001.
- ^ Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J (January 1993). "Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA". Science. 259 (5091): 94–97. Bibcode:1993Sci...259...94T. doi:10.1126/science.8418502. PMID 8418502.
- ^ a b Trojan J, Pan YX, Wei MX, Ly A, Shevelev A, Bierwagen M, et al. (2012). "Methodology for Anti-Gene Anti-IGF-I Therapy of Malignant Tumours". Chemotherapy Research and Practice. 2012: 1–12. doi:10.1155/2012/721873. PMC 3287029. PMID 22400112.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Abbott A (April 1992). "Gene therapy. Italians first to use stem cells". Nature. 356 (6369): 465. Bibcode:1992Natur.356..465A. doi:10.1038/356465a0. PMID 1560817.
- ^ Cavazzana-Calvo M, Thrasher A, Mavilio F (February 2004). "The future of gene therapy". Nature. 427 (6977): 779–781. Bibcode:2004Natur.427..779C. doi:10.1038/427779a. PMID 14985734.
- ^ S; Blakeslee, Ra (18 May 1993). "Treatment for 'Bubble Boy Disease'". The New York Times. ISSN 0362-4331. Retrieved 9 February 2018.
- ^ Stein, Rob (11 October 2010). "First patient treated in stem cell study". The Washington Post. Retrieved 10 November 2010.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Death Prompts FDA to Suspend Arthritis Gene Therapy Trial". Medpage Today. 27 July 2007. Retrieved 10 November 2010.
- ^ Stolberg, Sheryl Gay (22 January 2000). "Gene Therapy Ordered Halted At University". The New York Times. Retrieved 10 November 2010.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wilson, Jennifer Fisher (18 March 2002). "Murine Gene Therapy Corrects Symptoms of Sickle Cell Disease". The Scientist – Magazine of the Life Sciences. Retrieved 17 August 2010.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ St. Jude Children's Research Hospital (4 December 2008). "Gene Therapy Corrects Sickle Cell Disease In Laboratory Study". ScienceDaily. Retrieved 29 December 2012.
- ^ Penman, Danny (11 October 2002). "Subtle gene therapy tackles blood disorder". New Scientist. Retrieved 17 August 2010.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "DNA nanoballs boost gene therapy". New Scientist. 12 May 2002. Retrieved 17 August 2010.
- ^ Ananthaswamy, Anil (20 March 2003). "Undercover genes slip into the brain". New Scientist. Retrieved 17 August 2010.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Holmes, Bob (13 March 2003). "Gene therapy may switch off Huntington's". New Scientist. Retrieved 17 August 2010.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Ott MG, Schmidt M, Schwarzwaelder K, Stein S, Siler U, Koehl U, et al. (April 2006). "Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1". Nature Medicine. 12 (4): 401–409. doi:10.1038/nm1393. PMID 16582916.
- ^ Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L (May 2006). "Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer". Nature Medicine. 12 (5): 585–591. doi:10.1038/nm1398. PMID 16633348.
- ^ Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. (October 2006). "Cancer regression in patients after transfer of genetically engineered lymphocytes". Science. 314 (5796): 126–129. Bibcode:2006Sci...314..126M. doi:10.1126/science.1129003. PMC 2267026. PMID 16946036.
- ^ Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. (November 2006). "Gene transfer in humans using a conditionally replicating lentiviral vector". Proceedings of the National Academy of Sciences of the United States of America. 103 (46): 17372–17377. Bibcode:2006PNAS..10317372L. doi:10.1073/pnas.0608138103. PMC 1635018. PMID 17090675.
- ^ "Penn Medicine presents HIV gene therapy trial data at CROI 2009". EurekAlert!. 10 February 2009. Retrieved 19 November 2009.
- ^ "Gene therapy first for poor sight". BBC News. 1 May 2007. Retrieved 3 May 2010.
- ^ Dolgin, E. (2009). "Colour blindness corrected by gene therapy". Nature. doi:10.1038/news.2009.921.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, et al. (November 2009). "Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy". Science. 326 (5954): 818–823. Bibcode:2009Sci...326..818C. doi:10.1126/science.1171242. PMID 19892975.
- ^ Komáromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, et al. (July 2010). "Gene therapy rescues cone function in congenital achromatopsia". Human Molecular Genetics. 19 (13): 2581–2593. doi:10.1093/hmg/ddq136. PMC 2883338. PMID 20378608.
- ^ Cavazzana-Calvo M, Payen E, Negre O, Wang G, Hehir K, Fusil F, et al. (September 2010). "Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia". Nature. 467 (7313): 318–322. Bibcode:2010Natur.467..318C. doi:10.1038/nature09328. PMC 3355472. PMID 20844535.
- ^ Galanello R, Origa R (May 2010). "Beta-thalassemia". Orphanet Journal of Rare Diseases. 5: 11. doi:10.1186/1750-1172-5-11. PMC 2893117. PMID 20492708.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ a b Beals, Jacquelyn K. (16 September 2010). Gene Therapy Frees Beta-Thalassemia Patient From Transfusions for 2+ Years. Medscape.com (16 September 2010). Retrieved 15 December 2012.
- ^ a b Leboulch P (20 March 2013). "Five year outcome of lentiviral gene therapy for human beta-thalassemia, lessons and prospects". Thalassemia Reports. 3 (1s): 108.
- ^ a b Clinical trial number NCT01639690 for "β-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced With TNS9.3.55 a Lentiviral Vector Encoding the Normal Human β-Globin Gene" at ClinicalTrials.gov
- ^ Trojan An Aristizabal B, Jay LM, Castillo T, Penagos P, Trojan J. Testing of IGF-I biomarker in an ethical context. Adv Modern Oncol Res, 2(4); 2016, doi:10.18282/amor:v2:i4.58
- ^ Castillo T, Trojan A, Noguera MC, Jay ML, Crane C, Alvarez A, Melo G, Penagos PJ, Shevelev A, Aristizabal BH, Briceño I, Ayala A, Duc HT, Trojan J. Epidemiologic experience in elaboration of molecular biology technology for immunogene therapy (in Spanish). Rev Cien, 2 (25); 2016, doi:10.14483/udistrital.jour.RC.2016.25.a6
- ^ Rosenberg, Tina (29 May 2011) The Man Who Had HIV and Now Does Not, New York.
- ^ "Gene Therapy Turns Several Leukemia Patients Cancer Free. Will It Work for Other Cancers, Too?". Singularity Hub. 6 January 2014. Retrieved 7 January 2014.
- ^ Yang ZJ, Zhang YR, Chen B, Zhang SL, Jia EZ, Wang LS, et al. (July 2009). "Phase I clinical trial on intracoronary administration of Ad-hHGF treating severe coronary artery disease". Molecular Biology Reports. 36 (6): 1323–1329. doi:10.1007/s11033-008-9315-3. PMID 18649012.
- ^ Hahn W, Pyun WB, Kim DS, Yoo WS, Lee SD, Won JH, et al. (October 2011). "Enhanced cardioprotective effects by coexpression of two isoforms of hepatocyte growth factor from naked plasmid DNA in a rat ischemic heart disease model". The Journal of Gene Medicine. 13 (10): 549–555. doi:10.1002/jgm.1603. PMID 21898720.
- ^ AdisInsight Vascular endothelial growth factor gene therapy - HSCI Page accessed 5 June 2016
- ^ Eurolab. Neovasculogen listing in Eurolab Page accessed 4 August 2015
- ^ Deev RV, Bozo IY, Mzhavanadze ND, Voronov DA, Gavrilenko AV, Chervyakov YV, et al. (September 2015). "pCMV-vegf165 Intramuscular Gene Transfer is an Effective Method of Treatment for Patients With Chronic Lower Limb Ischemia". Journal of Cardiovascular Pharmacology and Therapeutics. 20 (5): 473–482. doi:10.1177/1074248415574336. PMID 25770117.
- ^ "On Cancer: Launch of Stem Cell Therapy Trial Offers Hope for Patients with Inherited Blood Disorder". Memorial Sloan-Kettering Cancer Center. 16 July 2012. Retrieved 15 December 2012.
- ^ Pollack, Andrew (20 July 2012) European Agency Backs Approval of a Gene Therapy, The New York Times.
- ^ First Gene Therapy Approved by European Commission Archived 5 November 2012 at the Wayback Machine. UniQure (2 November 2012). Retrieved 15 December 2012.
- ^ "Chiesi and uniQure delay Glybera launch to add data". Biotechnology. The Pharma Letter. 4 August 2014. Retrieved 28 August 2014.
- ^ Burger, Ludwig; Hirschler, Ben (26 November 2014). "First gene therapy drug sets million-euro price record". Reuters. Retrieved 30 March 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Whalen, Jeanne (2 November 2012). "Gene-Therapy Approval Marks Major Milestone". Wall Street Journal.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Morrison, Chris (3 March 2015). "$1-million price tag set for Glybera gene therapy". TradeSecrets.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gene therapy approved in Europe for first time
- ^ Regalado, Antonio (4 May 2016). "The World's Most Expensive Medicine Is a Bust". MIT Technology Review.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Bosely, Sarah (30 April 2013). "Pioneering gene therapy trials offer hope for heart patients". The Guardian. Retrieved 28 April 2014.
- ^ First gene therapy trial for heart failure begins in UK. The Physicians Clinic (8 September 2013) Archived 29 April 2014 at the Wayback Machine
- ^ Celladon Receives Breakthrough Therapy Designation From FDA for MYDICAR(R), Novel, First-in-Class Therapy in Development to Treat Heart Failure Archived 10 July 2015 at the Wayback Machine. New York Times (10 April 2014)
- ^ Fernàndez-Ruiz I (March 2016). "Gene therapy: No improvement in outcomes with gene therapy for heart failure". Nature Reviews. Cardiology. 13 (3): 122–123. doi:10.1038/nrcardio.2016.14. PMID 26843287.
- ^ Biffi A, Montini E, Lorioli L, Cesani M, Fumagalli F, Plati T, et al. (August 2013). "Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy". Science. 341 (6148): 1233158. doi:10.1126/science.1233158. PMID 23845948.
- ^ Aiuti A, Biasco L, Scaramuzza S, Ferrua F, Cicalese MP, Baricordi C, et al. (August 2013). "Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome". Science. 341 (6148): 1233151. doi:10.1126/science.1233151. PMC 4375961. PMID 23845947.
- ^ Gallagher, James (21 April 2015) Gene therapy: 'Tame HIV' used to cure disease BBC News, Health, Retrieved 21 April 2015
- ^ Malech HL, Ochs HD (April 2015). "An emerging era of clinical benefit from gene therapy". JAMA. 313 (15): 1522–1523. doi:10.1001/jama.2015.2055. PMID 25898049.
- ^ Gene therapy cure for children with 'bubble baby' disease. Science Daily (18 November 2014)
- ^ Gene therapy provides safe, long-term relief for patients with severe hemophilia B. Science Daily (20 November 2014)
- ^ MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, Clark KR, During MJ, Cremers FP, Black GC, Lotery AJ, Downes SM, Webster AR, Seabra MC (March 2014). "Retinal gene therapy in patients with choroideremia: initial findings from a phase 1/2 clinical trial". Lancet. 383 (9923): 1129–1137. doi:10.1016/S0140-6736(13)62117-0. PMC 4171740. PMID 24439297.
- ^ Beali, Abigail (25 January 2014) Gene therapy restores sight in people with eye disease The New Scientist. Retrieved 25 January 2014
- ^ Tebas P, Stein D, Tang WW, Frank I, Wang SQ, Lee G, et al. (March 2014). "Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV". The New England Journal of Medicine. 370 (10): 901–910. doi:10.1056/NEJMoa1300662. PMC 4084652. PMID 24597865.
- ^ Dvorsky, George (6 March 2014) Scientists Create Genetically Modified Cells That Protect Against HIV io9, Biotechnology. Retrieved 6 March 2014
- ^ Clinical trial number NCT02247843 for "Stem Cell Gene Therapy for Sickle Cell Disease" at ClinicalTrials.gov
- ^ Clinical trial number NCT00012545 for "Collection and Storage of Umbilical Cord Stem Cells for Treatment of Sickle Cell Disease" at ClinicalTrials.gov
- ^ "Ten things you might have missed Monday from the world of business". Boston Globe. 3 February 2015. Retrieved 13 February 2015.
- ^ Zimmer, Carl (9 March 2015). "Protection Without a Vaccine". The New York Times. Retrieved 30 March 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, et al. (March 2015). "AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges". Nature. 519 (7541): 87–91. Bibcode:2015Natur.519...87G. doi:10.1038/nature14264. PMC 4352131. PMID 25707797.
- ^ a b Wade, Nicholas (19 March 2015). "Scientists Seek Ban on Method of Editing the Human Genome". The New York Times. Retrieved 20 March 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Pollack, Andrew (3 March 2015). "A Powerful New Way to Edit DNA". The New York Times. Retrieved 20 March 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Baltimore D, Berg P, Botchan M, Carroll D, Charo RA, Church G, et al. (April 2015). "Biotechnology. A prudent path forward for genomic engineering and germline gene modification". Science. 348 (6230): 36–38. Bibcode:2015Sci...348...36B. doi:10.1126/science.aab1028. PMC 4394183. PMID 25791083.
- ^ a b Lanphier E, Urnov F, Haecker SE, Werner M, Smolenski J (March 2015). "Don't edit the human germ line". Nature. 519 (7544): 410–411. Bibcode:2015Natur.519..410L. doi:10.1038/519410a. PMID 25810189.
- ^ Henry, Robin (19 February 2017). "Leukaemia cure hopes rise as girl is gene‑edited". The Times. Retrieved 20 February 2017.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Sample, Ian (5 November 2015). "Baby girl is first in the world to be treated with 'designer immune cells'". The Guardian. Retrieved 6 November 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Wade, Nicholas (3 December 2015). "Scientists Place Moratorium on Edits to Human Genome That Could Be Inherited". The New York Times. Retrieved 3 December 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Walsh, Fergus (3 December 2015). "Gene editing: Is era of designer humans getting closer?". BBC News Health. Retrieved 31 December 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Summary of opinion1 (initial authorisation) Strimvelis" (PDF). European Medicines Agency. 1 April 2016. pp. 1–2. Retrieved 13 April 2016.
- ^ Hirscheler, Ben (1 April 2016). "Europe gives green light to first gene therapy for children". Reuters. Retrieved 13 April 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Reeves, Rachel (6 June 2016). "Second gene therapy wins approval in Europe". Bionews. Retrieved 20 February 2017.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Coghlan, Andy (9 April 2016). "Gene Therapy Approved". The New Scientist. No. 3068. pp. 8–9.
{{cite magazine}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Cyranoski D (July 2016). "Chinese scientists to pioneer first human CRISPR trial". Nature. 535 (7613): 476–477. Bibcode:2016Natur.535..476C. doi:10.1038/nature.2016.20302. PMID 27466105.
- ^ Bennett, Jay (15 November 2016). "Chinese Scientists Become First to Use CRISPR Gene-Editing on Humans". Popular Mechanics. Retrieved 16 November 2016.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Lee TW, Southern KW, Perry LA, Penny-Dimri JC, Aslam AA (June 2016). "Topical cystic fibrosis transmembrane conductance regulator gene replacement for cystic fibrosis-related lung disease". The Cochrane Database of Systematic Reviews (6): CD005599. doi:10.1002/14651858.CD005599.pub5. PMID 27314455.
- ^ Whipple, Tom (1 March 2017). "New gene therapy 'shrinks tumours like ice cubes'". The Times. Retrieved 1 March 2017.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Coghlan, Andy (March 2017). "Gene therapy 'cures' boy of blood disease that affects millions". New Scientist.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "FDA approval brings first gene therapy to the United States". FDA News Release. U.S. Food and Drug Administration. 30 August 2017.
- ^ Research, Center for Drug Evaluation and. "Approved Drugs - FDA approves axicabtagene ciloleucel for large B-cell lymphoma". www.fda.gov. Retrieved 5 January 2018.
- ^ Rangarajan S, Walsh L, Lester W, Perry D, Madan B, Laffan M, et al. (December 2017). "AAV5-Factor VIII Gene Transfer in Severe Hemophilia A". The New England Journal of Medicine. 377 (26): 2519–2530. doi:10.1056/nejmoa1708483. hdl:10044/1/57163. PMID 29224506.
- ^ van den Berg HM (December 2017). "A Cure for Hemophilia within Reach". The New England Journal of Medicine. 377 (26): 2592–2593. doi:10.1056/nejme1713888. PMID 29224412.
- ^ Office of the Commissioner (19 December 2017). "FDA approves novel gene therapy to treat patients with a rare form of inherited vision loss". FDA. Retrieved 20 December 2017.
- ^ Herper, Matthew (3 January 2018). "Spark Therapeutics Sets Price Of Blindness-Treating Gene Therapy At $850,000". Forbes. Retrieved 4 January 2018.
- ^ Sheridan, Kate (19 December 2017). "The FDA approved a gene therapy to treat blindness in a groundbreaking moment for DNA-based medicine". Newsweek. Retrieved 20 December 2017.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Olowoyeye, Abiola; Okwundu, Charles I. (2018). "Gene therapy for sickle cell disease". The Cochrane Database of Systematic Reviews. 11: CD007652. doi:10.1002/14651858.CD007652.pub6. ISSN 1469-493X. PMC 6517046. PMID 30480767.
- ^ Marchione, Marilyn (7 February 2019). "Tests suggest scientists achieved 1st 'in body' gene editing". AP News. Retrieved 7 February 2019.
- ^ Staff (2 February 2019). "Ascending Dose Study of Genome Editing by the Zinc Finger Nuclease (ZFN) Therapeutic SB-913 in Subjects With MPS II". ClinicalTrials.gov. U.S. National Library of Medicine. Retrieved 7 February 2019.
- ^ Stein, Rob (24 May 2019). "At $2.1 Million, New Gene Therapy Is The Most Expensive Drug Ever". NPR. Retrieved 24 May 2019.
- ^ Tong, Amber (3 June 2019). "EU stamps historic OK on bluebird's gene therapy for β-thalassemia — now sit back and wait for the price". Retrieved 4 June 2019.
- ^ "Single Ascending Dose Study in Participants With LCA10 - Full Text View - ClinicalTrials.gov". clinicaltrials.gov. Retrieved 20 August 2019.
- ^ "Allergan and Editas Medicine Initiate the Brilliance Phase 1/2 Clinical Trial of AGN-151587 (EDIT-101) for the Treatment of LCA10". Editas Medicine. Retrieved 20 August 2019.
- ^ "WADA Gene Doping". WADA. Archived from the original on 21 November 2009. Retrieved 27 September 2013.
- ^ Kayser B, Mauron A, Miah A (March 2007). "Current anti-doping policy: a critical appraisal". BMC Medical Ethics. 8: 2. doi:10.1186/1472-6939-8-2. PMC 1851967. PMID 17394662.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Powell R, Buchanan A (February 2011). "Breaking evolution's chains: the prospect of deliberate genetic modification in humans". The Journal of Medicine and Philosophy. 36 (1): 6–27. doi:10.1093/jmp/jhq057. PMID 21228084.
- ^ Baylis F, Robert JS (2004). "The inevitability of genetic enhancement technologies". Bioethics. 18 (1): 1–26. doi:10.1111/j.1467-8519.2004.00376.x. PMID 15168695.
- ^ Evans, John (2002). Playing God?: Human Genetic Engineering and the Rationalization of Public Bioethical Debate. University of Chicago Press. ISBN 978-0-226-22262-2.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Gene Therapy and Genetic Engineering. Archived 3 December 2013 at the Wayback Machine The Center for Health Ethics, University of Missouri School of Medicine. 25 April 2013.
- ^ Roco MC, Bainbridge WS (2002). "Converging Technologies for Improving Human Performance: Integrating From the Nanoscale". Journal of Nanoparticle Research. 4 (4): 281–295. Bibcode:2002JNR.....4..281R. doi:10.1023/A:1021152023349.
- ^ Allhoff F (March 2005). "Germ-line genetic enhancement and Rawlsian primary goods" (PDF). Kennedy Institute of Ethics Journal. 15 (1): 39–56. CiteSeerX 10.1.1.566.171. doi:10.1353/ken.2005.0007. PMID 15881795.
- ^ Ranisch R (January 2020). "Special Issue:Human Germline Editing". Bioethics. 34 (1): 1–143.
- ^ "Ethical issues related to prenatal genetic testing. The Council on Ethical and Judicial Affairs, American Medical Association". Archives of Family Medicine. 3 (7): 633–642. July 1994. doi:10.1001/archfami.3.7.633. PMID 7921302.
- ^ The Declaration of Inuyama: Human Genome Mapping, Genetic Screening and Gene Therapy. cioms.ch
- ^ Smith KR, Chan S, Harris J (October 2012). "Human germline genetic modification: scientific and bioethical perspectives". Archives of Medical Research. 43 (7): 491–513. doi:10.1016/j.arcmed.2012.09.003. PMID 23072719.
- ^ Reardon, Sara (14 February 2017). "US science advisers outline path to genetically modified babies". Nature. doi:10.1038/nature.2017.21474.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Liang P, Xu Y, Zhang X, Ding C, Huang R, Zhang Z, Lv J, Xie X, Chen Y, Li Y, Sun Y, Bai Y, Songyang Z, Ma W, Zhou C, Huang J (May 2015). "CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes". Protein & Cell. 6 (5): 363–372. doi:10.1007/s13238-015-0153-5. PMC 4417674. PMID 25894090.
- ^ Kolata, Gina (23 April 2015). "Chinese Scientists Edit Genes of Human Embryos, Raising Concerns". The New York Times. Retrieved 24 April 2015.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Harmon, Amy (14 February 2017). "Human Gene Editing Receives Science Panel's Support". The New York Times. ISSN 0362-4331. Retrieved 17 February 2017.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Committee on Human Gene Editing: Scientific, Medical, and Ethical Considerations. "Human Genome Editing: Science, Ethics, and Governance". nationalacademies.org. National Academy of Sciences; National Academy of Medicine. Retrieved 21 February 2017.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ "Scientists OK genetically engineering babies". New York Post. Reuters. 14 February 2017. Retrieved 17 February 2017.
- ^ Human Genome Organization. HUGO Ethics Committee (April 2001). Statement on Gene Therapy Research.
- ^ Isasi, Rosario M.; Nguyen, Thu Minh; Knoppers, Bartha M. (October 2006). "National Regulatory Frameworks Regarding Human Genetic Modification Technologies (Somatic and Germline Modification)" (PDF). Genetics & Public Policy Center. Archived from the original (PDF) on 17 September 2014.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b National Institutes of Health. NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules. Revised April 2016.
- ^ U.S. Department of Health & Human Services. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. The Belmont Report: Ethical Principles and Guidelines for the Protection of Human Subjects of Research. 18 April 1979.
- ^ U.S. Food and Drug Administration (14 October 1993). "Application of Current Statutory Authorities to Human Somatic Cell Therapy Products and Gene Therapy Products" (PDF). Federal Register. 58 (197).
- ^ U.S. Department of Health and Human Services. Food and Drug Administration. Center for Biologics Evaluation and Research. Guidance for Industry: Guidance for Human Somatic Cell Therapy and Gene Therapy. March 1998.
- ^ "A Real-life 'I Am Legend?' Researcher Champions Development Of 'Reovirus' As Potential Treatment For Cancer". Sciencedaily.com. 9 May 2008. Retrieved 17 August 2010.
- ^ Will Gene Therapy Change the Human Race? at IMDb
- ^ Cass, Stephen (15 September 2008). "Stargate Atlantis: Gene Therapy". Discover. Retrieved 14 December 2016.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Beeler, Stan; Dickson, Lisa (11 July 2006). Reading Stargate SG-1. I.B.Tauris. ISBN 978-1-84511-183-0.
Further reading
- Tinkov S, Bekeredjian R, Winter G, Coester C (20 November 2000). "Polyplex-conjugated microbubbles for enhanced ultrasound targeted gene therapy" (PDF). Georgia World Congress Center, Atlanta, GA, USA: 2008 AAPS Annual Meeting and Exposition. Archived from the original (PDF) on 7 July 2012. Retrieved 26 January 2009.
- Gardlík R, Pálffy R, Hodosy J, Lukács J, Turna J, Celec P (April 2005). "Vectors and delivery systems in gene therapy". Medical Science Monitor. 11 (4): RA110–121. PMID 15795707.
- Staff (18 November 2005). "Gene Therapy" (FAQ). Human Genome Project Information. Oak Ridge National Laboratory. Retrieved 28 May 2006.
- Salmons B, Günzburg WH (April 1993). "Targeting of retroviral vectors for gene therapy". Human Gene Therapy. 4 (2): 129–141. doi:10.1089/hum.1993.4.2-129. PMID 8494923.
- Baum C, Düllmann J, Li Z, Fehse B, Meyer J, Williams DA, von Kalle C (March 2003). "Side effects of retroviral gene transfer into hematopoietic stem cells". Blood. 101 (6): 2099–2114. doi:10.1182/blood-2002-07-2314. PMID 12511419.
- Horn PA, Morris JC, Neff T, Kiem HP (September 2004). "Stem cell gene transfer—efficacy and safety in large animal studies". Molecular Therapy. 10 (3): 417–431. doi:10.1016/j.ymthe.2004.05.017. PMID 15336643.
- Wang H, Shayakhmetov DM, Leege T, Harkey M, Li Q, Papayannopoulou T, Stamatoyannopolous G, Lieber A (September 2005). "A capsid-modified helper-dependent adenovirus vector containing the beta-globin locus control region displays a nonrandom integration pattern and allows stable, erythroid-specific gene expression". Journal of Virology. 79 (17): 10999–11013. doi:10.1128/JVI.79.17.10999-11013.2005. PMC 1193620. PMID 16103151.
