Xanthonoid
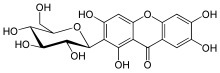
A xanthonoid is a chemical natural phenolic compound formed from the xanthone backbone. Many members of the Clusiaceae contain xanthonoids.
Xanthonoid biosynthesis in cell cultures of Hypericum androsaemum involves the presence of a benzophenone synthase condensing a molecule of benzoyl-CoA with three malonyl-CoA yielding to 2,4,6-trihydroxybenzophenone. This intermediate is subsequently converted by a benzophenone 3′-hydroxylase, a cytochrome P450 monooxygenase, leading to the formation of 2,3′,4,6-tetrahydroxybenzophenone.[1]
Some examples are tomentonone, zeyloxanthonone and calozeyloxanthone isolated from the bark of Calophyllum tomentosum,[2] apetalinone A, B, C and D from Calophyllum apetalum,[3] gaudichaudione A, B, C, D, E, F, G, H, gaudichaudiic acid A, B, C, D, E, morellic acid and forbesione from Garcinia gaudichaudii,[4] methylswertianin and bellidifolin from Swertia punicea[5] or psorospermin obtained from Psorospermum febrifugum.[6] Cassiaxanthone can be found in Cassia reticulata.[7]
Cytotoxic xanthones (gambogin, morellin dimethyl acetal, isomoreollin B, moreollic acid, gambogenic acid, gambogenin, isogambogenin, desoxygambogenin, gambogenin dimethyl acetal, gambogellic acid, gambogic acid, isomorellin, morellic acid, desoxymorellin and hanburin) can be isolated from the dry latex of Garcinia hanburyi (gamboge tree).[8]
References
[edit]- ^ Alternative pathways of xanthone biosynthesis in cell cultures of Hypericum androsaemum L. Werner Schmidt and Ludger Beerhues, FEBS Letters, Volume 420, Issues 2-3, 29 December 1997, Pages 143-146, doi:10.1016/S0014-5793(97)01507-X
- ^ Tomentonone, a new xanthonoid from the stem bark of Calophyllum tomentosum. Banerji A., Deshpande A. D., Prabhu B. R. and Padmanava P., Journal of natural products, 1994, vol. 57, no3, pp. 396-399
- ^ Prenylated xanthonoids from Calophyllum apetalum. Munekazu Iinuma, Tetsuro Ito, Hideki Tosa, Toshiyuki Tanaka, Ryoko Miyake and Veliah Chelladurai, Phytochemistry, Volume 46, Issue 8, December 1997, Pages 1423-1429, doi:10.1016/S0031-9422(97)00507-4
- ^ Novel cytotoxic polyprenylated xanthonoids from Garcinia gaudichaudii (Guttiferae). Shu-Geng Cao, Valerie H. L. Sng, Xiao-Hua Wu, a, Keng-Yeow Sim, B. H. K. Tan, J. T. Pereira and S. H. Goh, Tetrahedron, Volume 54, Issue 36, 3 September 1998, Pages 10915-10924, doi:10.1016/S0040-4020(98)00644-9
- ^ Anti-diabetic effect of methylswertianin and bellidifolin from Swertia punicea Hemsl. and its potential mechanism. L.-Y. Tian, X. Bai, X.-H. Chen, J.-B. Fang, S.-H. Liu and J.-C. Chen, Phytomedicine, Volume 17, Issue 7, June 2010, Pages 533-539, doi:10.1016/j.phymed.2009.10.007
- ^ Psorospermin, A New Antileukemic Xanthone From Psorospermum febrifugum. Kupchan S. Morris, Streelman David R. and Sneden Albert T., Journal of Natural Products, Volume 43, issue 2 (1980), p. 296-301. doi:10.1021/np50008a010
- ^ Cassiaxanthone, a hydroxyxanthone dicarboxylic acid from Cassia species. M. S. R. Nair, T. C. McMorris and Marjorie Anchel, Phytochemistry, Volume 9, Issue 5, May 1970, Pages 1153-1155, doi:10.1016/S0031-9422(00)85246-2
- ^ Cytotoxic xanthones from Garcinia hanburyi. Jun Asano, Kazuhiro Chiba, Masahiro Tada and Takao Yoshii, Phytochemistry, Volume 41, Issue 3, February 1996, Pages 815-820, doi:10.1016/0031-9422(95)00682-6
