Boron trioxide
![Crystal structure of B2O3 [1]](http://upload.wikimedia.org/wikipedia/commons/thumb/d/d9/B2O3powder.JPG/220px-B2O3powder.JPG)
| |
| |
| Names | |
|---|---|
| Other names
boron oxide, diboron trioxide, boron sesquioxide, boric oxide, boria
Boric acid anhydride | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.013.751 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| B2O3 | |
| Molar mass | 69.6182 g/mol |
| Appearance | white, glassy solid |
| Density | 2.460 g/cm3, liquid; 2.55 g/cm3, trigonal; |
| Melting point | 450 °C (842 °F; 723 K) (trigonal) 510 °C (tetrahedral) |
| Boiling point | 1,860 °C (3,380 °F; 2,130 K) ,[2] sublimates at 1500 °C[3] |
| 1.1 g/100mL (10 °C) 3.3 g/100mL (20 °C) 15.7 100 g/100mL (100 °C) | |
| Solubility | partially soluble in methanol |
| Acidity (pKa) | ~ 4 |
| Thermochemistry | |
Heat capacity (C)
|
66.9 J/mol K |
Std molar
entropy (S⦵298) |
80.8 J/mol K |
Std enthalpy of
formation (ΔfH⦵298) |
-1254 kJ/mol |
Gibbs free energy (ΔfG⦵)
|
-832 kJ/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Template:Hazchem Xi[4] |
| NFPA 704 (fire diamond) | |
| Flash point | noncombustible |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
3163 mg/kg (oral, mouse)[5] |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3[4] |
REL (Recommended)
|
TWA 10 mg/m3[4] |
IDLH (Immediate danger)
|
2000 mg/m3[4] |
| Supplementary data page | |
| Boron trioxide (data page) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Boron trioxide (or diboron trioxide) is one of the oxides of boron. It is a white, glassy solid with the formula B2O3. It is almost always found as the vitreous (amorphous) form; however, it can be crystallized after extensive annealing (that is, under prolonged heat).
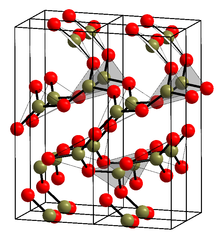
Glassy boron oxide (g-B2O3) is thought to be composed of boroxol rings which are six-membered rings composed of alternating 3-coordinate boron and 2-coordinate oxygen. Because of the difficulty of building disordered models at the correct density with a large number of boroxol rings, this view was initially controversial, but such models have recently been constructed and exhibit spectroscopic properties in excellent agreement with experiment.[6] The rings are thought to make a few BO3 triangles, but mostly link (polymerize) into ribbons and sheets.[7][8] The crystalline form (α-B2O3) (see structure in the infobox[1]) is exclusively composed of BO3 triangles. This trigonal, quartz-like network undergoes a coesite-like transformation to monoclinic β-B2O3 at several gigapascals (9.5 GPa).[9]
Preparation
Boron trioxide is produced by treating borax with sulfuric acid in a fusion furnace. At temperatures above 750 °C, the molten boron oxide layer separates out from sodium sulfate. It is then decanted, cooled and obtained in 96–97% purity.[3]
Another method is heating boric acid above ~300 °C. Boric acid will initially decompose into water steam and metaboric acid (HBO2) at around 170 °C, and further heating above 300 °C will produce more steam and boron trioxide. The reactions are:
- H3BO3 → HBO2 + H2O
- 2 HBO2 → B2O3 + H2O
Boric acid goes to anhydrous microcrystalline B2O3 in a heated fluidized bed.[10] Carefully controlled heating rate avoids gumming as water evolves. Molten boron oxide attacks silicates. Internally graphitized tubes via acetylene thermal decomposition are passivated.[11]
Crystallization of molten α-B2O3 at ambient pressure is strongly kinetically disfavored (compare liquid and crystal densities). Threshold conditions for crystallization of the amorphous solid are 10 kbar and ~200 °C.[12] Its proposed crystal structure in enantiomorphic space groups P31(#144); P32(#145)[13][14] (e.g., γ-glycine) has been revised to enantiomorphic space groups P3121(#152); P3221(#154)[15](e.g., α-quartz).
Boron oxide will also form when diborane (B2H6) reacts with oxygen in the air or trace amounts of moisture:
- 2B2H6(g) + 3O2(g) → 2B2O3(s) + 6H2(g)
- B2H6(g) + 3H2O(g) → B2O3(s) + 6H2(g)[16]
Applications
- Fluxing agent for glass and enamels
- Starting material for synthesizing other boron compounds such as boron carbide
- An additive used in glass fibres (optical fibres)
- It is used in the production of borosilicate glass
- The inert capping layer in the LEC process for the production of gallium arsenide single crystal
- As an acid catalyst in organic synthesis
See also
References
- ^ a b Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The Crystal Structure of Trigonal Diboron Trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ High temperature corrosion and materials chemistry: proceedings of the Per Kofstad Memorial Symposium. Proceedings of the Electrochemical Society. The Electrochemical Society. 2000. p. 496. ISBN 1-56677-261-3.
- ^ a b Patnaik, P. (2003). Handbook of Inorganic Chemical Compounds. McGraw-Hill. p. 119. ISBN 0-07-049439-8. Retrieved 2009-06-06.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0060". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Boron oxide". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Ferlat, G.; Charpentier, T.; Seitsonen, A. P.; Takada, A.; Lazzeri, M.; Cormier, L.; Calas, G.; Mauri. F. (2008). "Boroxol Rings in Liquid and Vitreous B2O3 from First Principles". Phys. Rev. Lett. 101: 065504. doi:10.1103/PhysRevLett.101.065504.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Eckert, H. (1992). "Structural characterization of noncrystalline solids and glasses using solid state NMR". Progress in Nuclear Magnetic Resonance Spectroscopy. 24 (3): 159–293. doi:10.1016/0079-6565(92)80001-V.
- ^ Hwang, S.-J.; Fernandez, C.; Amoureux, J. P.; Cho, J.; Martin, S. W.; Pruski, M. (1997). "Quantitative study of the short range order in B2O3 and B2S3 by MAS and two-dimensional triple-quantum MAS 11B NMR". Solid State Nuclear Magnetic Resonance. 8 (2): 109–121. doi:10.1016/S0926-2040(96)01280-5. PMID 9203284.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Brazhkin, V. V.; Katayama, Y.; Inamura, Y.; Kondrin, M. V.; Lyapin, A. G.; Popova, S. V.; Voloshin, R. N. (2003). "Structural transformations in liquid, crystalline and glassy B2O3 under high pressure". JETP Letters. 78 (6): 393–397. doi:10.1134/1.1630134.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kocakuşak, S.; Akçay, K.; Ayok, T.; Koöroğlu, H. J.; Koral, M.; Savaşçi, Ö. T.; Tolun, R. (1996). "Production of anhydrous, crystalline boron oxide in fluidized bed reactor". Chemical Engineering and Processing. 35 (4): 311–317. doi:10.1016/0255-2701(95)04142-7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Morelock, C. R. (1961). "Research Laboratory Report #61-RL-2672M" (Document). General Electric.
- ^ Aziz, M. J.; Nygren, E.; Hays, J. F.; Turnbull, D. (1985). "Crystal Growth Kinetics of Boron Oxide Under Pressure". Journal of Applied Physics. 57 (6): 2233. doi:10.1063/1.334368.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gurr, G. E.; Montgomery, P. W.; Knutson, C. D.; Gorres, B. T. (1970). "The crystal structure of trigonal diboron trioxide". Acta Crystallographica B. 26 (7): 906–915. doi:10.1107/S0567740870003369.
- ^ Strong, S. L.; Wells, A. F.; Kaplow, R. (1971). "On the crystal structure of B2O3". Acta Crystallographica B. 27 (8): 1662–1663. doi:10.1107/S0567740871004515.
- ^ Effenberger, H.; Lengauer, C. L.; Parthé, E. (2001). "Trigonal B2O3 with Higher Space-Group Symmetry: Results of a Reevaluation". Monatshefte für Chemie. 132 (12): 1515–1517. doi:10.1007/s007060170008.
- ^ AirProducts (2011). "Diborane Storage & Delivery" (Document).
{{cite document}}: Cite document requires|publisher=(help); Unknown parameter|url=ignored (help)

