Bcl-2
Bcl-2, encoded in humans by the BCL2 gene, is the founding member of the Bcl-2 family of regulator proteins. BCL2 blocks programmed cell death (apoptosis) [5] while other BCL2 family members can either inhibit or induce it.[6][7] It was the first apoptosis regulator identified in any organism.[8]
Bcl-2 derives its name from B-cell lymphoma 2, as it is the second member of a range of proteins initially described in chromosomal translocations involving chromosomes 14 and 18 in follicular lymphomas. Orthologs[9] (such as Bcl2 in mice) have been identified in numerous mammals for which complete genome data are available.
Like BCL3, BCL5, BCL6, BCL7A, BCL9, and BCL10, it has clinical significance in lymphoma.
Isoforms
[edit]The two isoforms of Bcl-2, Isoform 1, and Isoform 2, exhibit a similar fold. However, results in the ability of these isoforms to bind to the BAD and BAK proteins, as well as in the structural topology and electrostatic potential of the binding groove, suggest differences in antiapoptotic activity for the two isoforms.[10]
Function
[edit]BCL-2 is localized to the outer membrane of mitochondria, where it plays an important role in promoting cellular survival and inhibiting the actions of pro-apoptotic proteins. The pro-apoptotic proteins in the BCL-2 family, including Bax and Bak, normally act on the mitochondrial membrane to promote permeabilization and release of cytochrome c and ROS, that are important signals in the apoptosis cascade. These pro-apoptotic proteins are in turn activated by BH3-only proteins, and are inhibited by the function of BCL-2 and its relative BCL-Xl.[11]
There are additional non-canonical roles of BCL-2 that are being explored. BCL-2 is known to regulate mitochondrial dynamics, and is involved in the regulation of mitochondrial fusion and fission. Additionally, in pancreatic beta-cells, BCL-2 and BCL-Xl are known to be involved in controlling metabolic activity and insulin secretion, with inhibition of BCL-2/Xl showing increasing metabolic activity,[12] but also additional ROS production; this suggests it has a protective metabolic effect in conditions of high demand.[13]
Role in disease
[edit]Damage to the Bcl-2 gene has been identified as a cause of a number of cancers, including melanoma, breast, prostate, chronic lymphocytic leukemia, and lung cancer, and a possible cause of schizophrenia and autoimmunity. It is also a cause of resistance to cancer treatments.[14]
Cancer
[edit]Cancer can be seen as a disturbance in the homeostatic balance between cell growth and cell death. Over-expression of anti-apoptotic genes, and under-expression of pro-apoptotic genes, can result in the lack of cell death that is characteristic of cancer. An example can be seen in lymphomas. The over-expression of the anti-apoptotic Bcl-2 protein in lymphocytes alone does not cause cancer. But simultaneous over-expression of Bcl-2 and the proto-oncogene myc may produce aggressive B-cell malignancies including lymphoma.[15] In follicular lymphoma, a chromosomal translocation commonly occurs between the fourteenth and the eighteenth chromosomes – t(14;18) – which places the Bcl-2 gene from chromosome 18 next to the immunoglobulin heavy chain locus on chromosome 14. This fusion gene is deregulated, leading to the transcription of excessively high levels of Bcl-2.[16] This decreases the propensity of these cells for apoptosis. Bcl-2 expression is frequent in small cell lung cancer, accounting for 76% cases in one study.[17]
Auto-immune diseases
[edit]Apoptosis plays an active role in regulating the immune system. When it is functional, it can cause immune unresponsiveness to self-antigens via both central and peripheral tolerance. In the case of defective apoptosis, it may contribute to etiological aspects of autoimmune diseases.[18] The autoimmune disease type 1 diabetes can be caused by defective apoptosis, which leads to aberrant T cell AICD and defective peripheral tolerance. Due to the fact that dendritic cells are the immune system's most important antigen-presenting cells, their activity must be tightly regulated by mechanisms such as apoptosis. Researchers have found that mice containing dendritic cells that are Bim -/-, thus unable to induce effective apoptosis, have autoimmune diseases more so than those that have normal dendritic cells.[18] Other studies have shown that dendritic cell lifespan may be partly controlled by a timer dependent on anti-apoptotic Bcl-2.[18]
Other
[edit]Apoptosis plays an important role in regulating a variety of diseases. For example, schizophrenia is a psychiatric disorder in which an abnormal ratio of pro- and anti-apoptotic factors may contribute towards pathogenesis.[19] Some evidence suggests that this may result from abnormal expression of Bcl-2 and increased expression of caspase-3.[19]
Diagnostic use
[edit]Antibodies to Bcl-2 can be used with immunohistochemistry to identify cells containing the antigen. In healthy tissue, these antibodies react with B-cells in the mantle zone, as well as some T-cells. However, positive cells increase considerably in follicular lymphoma, as well as many other forms of cancer. In some cases, the presence or absence of Bcl-2 staining in biopsies may be significant for the patient's prognosis or likelihood of relapse.[20]
Targeted therapies
[edit]Targeted and selective Bcl-2 inhibitors that have been in development or are currently in the clinic include:
Oblimersen
[edit]An antisense oligonucleotide drug, oblimersen (G3139), was developed by Genta Incorporated to target Bcl-2. An antisense DNA or RNA strand is non-coding and complementary to the coding strand (which is the template for producing respectively RNA or protein). An antisense drug is a short sequence of RNA that hybridises with and inactivates mRNA, preventing the protein from being formed.
Human lymphoma cell proliferation (with t(14;18) translocation) could be inhibited by antisense RNA targeted at the start codon region of Bcl-2 mRNA. In vitro studies led to the identification of Genasense, which is complementary to the first 6 codons of Bcl-2 mRNA.[21]
These showed successful results in Phase I/II trials for lymphoma. A large Phase III trial was launched in 2004.[22] As of 2016, the drug had not been approved and its developer was out of business.[23]
ABT-737 and navitoclax (ABT-263)
[edit]In the mid-2000s, Abbott Laboratories developed a novel inhibitor of Bcl-2, Bcl-xL and Bcl-w, known as ABT-737. This compound is part of a group of BH3 mimetic small molecule inhibitors (SMI) that target these Bcl-2 family proteins, but not A1 or Mcl-1. ABT-737 is superior to previous BCL-2 inhibitors given its higher affinity for Bcl-2, Bcl-xL and Bcl-w. In vitro studies showed that primary cells from patients with B-cell malignancies are sensitive to ABT-737.[24]
In animal models, it improves survival, causes tumor regression and cures a high percentage of mice.[25] In preclinical studies utilizing patient xenografts, ABT-737 showed efficacy for treating lymphoma and other blood cancers.[26] Because of its unfavorable pharmacologic properties ABT-737 is not appropriate for clinical trials, while its orally bioavailable derivative navitoclax (ABT-263) has similar activity on small cell lung cancer (SCLC) cell lines and has entered clinical trials.[27] While clinical responses with navitoclax were promising, mechanistic dose-limiting thrombocytopenia was observed in patients under treatment due to Bcl-xL inhibition in platelets.[28][29][30]
Venetoclax (ABT-199)
[edit]Due to dose-limiting thrombocytopenia of navitoclax as a result of Bcl-xL inhibition, Abbvie successfully developed the highly selective inhibitor venetoclax (ABT-199), which inhibits Bcl-2, but not Bcl-xL or Bcl-w.[31] Clinical trials studied the effects of venetoclax, a BH3-mimetic drug designed to block the function of the Bcl-2 protein, on patients with chronic lymphocytic leukemia (CLL).[32][33] Good responses have been reported and thrombocytopenia was no longer observed.[33][34] A phase 3 trial started in Dec 2015.[35] It was approved by the US FDA in April 2016 as a second-line treatment for CLL associated with 17-p deletion.[36] This was the first FDA approval of a BCL-2 inhibitor.[36] In June 2018, the FDA broadened the approval for anyone with CLL or small lymphocytic lymphoma, with or without 17p deletion, still as a second-line treatment.[37]
Sonrotoclax (BGB-11417)
[edit]Venetoclax drug resistance has been noted with the G101V mutation in BCL-2 observed in relapsing patients.[38] Sonrotoclax shows greater tumor growth inhibition in hematologic tumor models than venetoclax and inhibits venetoclax-resistant BCL-2 variants. Sonrotoclax is under clinical investigation as a monotherapy and in combination with other anticancer agents.[39]
Interactions
[edit]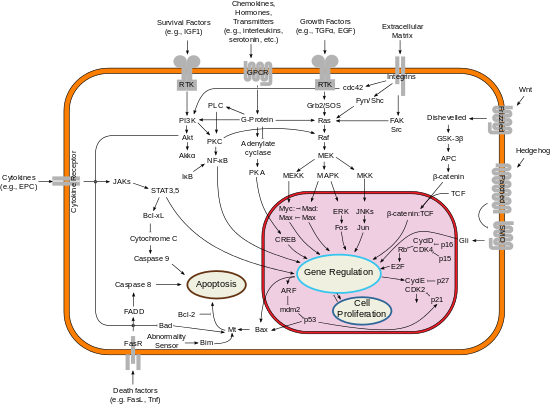
Bcl-2 has been shown to interact with:
- BAK1,[40][41]
- BCAP31,[42]
- BCL2-like 1,[40][43]
- BCL2L11,[44][45][46]
- BECN1,[47]
- BID,[44][48]
- BMF,[49]
- BNIP2,[50][51]
- BNIP3,[51][52]
- BNIPL,[50][53]
- BAD[44][54]
- BAX,[40][55][56][57]
- BIK,[44][58]
- C-Raf,[59]
- CAPN2,[60]
- CASP8,[61][62]
- Cdk1,[63][64]
- HRK,[44][65]
- IRS1,[66]
- Myc,[67]
- NR4A1,[40]
- Noxa,[44][68]
- PPP2CA,[69]
- PSEN1,[70]
- RAD9A,[55]
- RRAS,[71]
- RTN4,[72]
- SMN1,[73]
- SOD1,[74] and
- TP53BP2.[75]
See also
[edit]References
[edit]- ^ a b c GRCh38: Ensembl release 89: ENSG00000171791 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000057329 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ (November 1990). "Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death". Nature. 348 (6299): 334–336. Bibcode:1990Natur.348..334H. doi:10.1038/348334a0. PMID 2250705.
- ^ Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM (November 1984). "Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation". Science. 226 (4678): 1097–1099. Bibcode:1984Sci...226.1097T. doi:10.1126/science.6093263. PMID 6093263.
- ^ Cleary ML, Smith SD, Sklar J (October 1986). "Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation". Cell. 47 (1): 19–28. doi:10.1016/0092-8674(86)90362-4. PMID 2875799. S2CID 31493780.
- ^ Kelly GL, Strasser A (2020). "Toward Targeting Antiapoptotic MCL-1 for Cancer Therapy". Annual Review of Cancer Biology. 4: 299–313. doi:10.1146/annurev-cancerbio-030419-033510. hdl:11343/252362.
- ^ "OrthoMaM phylogenetic marker: Bcl-2 coding sequence". Archived from the original on 24 September 2015. Retrieved 20 December 2009.
- ^ PDB: 1G5M; Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, et al. (March 2001). "Solution structure of the antiapoptotic protein bcl-2". Proceedings of the National Academy of Sciences of the United States of America. 98 (6): 3012–3017. Bibcode:2001PNAS...98.3012P. doi:10.1073/pnas.041619798. PMC 30598. PMID 11248023.
- ^ Hardwick JM, Soane L (February 2013). "Multiple functions of BCL-2 family proteins". Cold Spring Harbor Perspectives in Biology. 5 (2): a008722. doi:10.1101/cshperspect.a008722. PMC 3552500. PMID 23378584.
- ^ Luciani DS, White SA, Widenmaier SB, Saran VV, Taghizadeh F, Hu X, et al. (January 2013). "Bcl-2 and Bcl-xL suppress glucose signaling in pancreatic β-cells". Diabetes. 62 (1): 170–182. doi:10.2337/db11-1464. PMC 3526034. PMID 22933114.
- ^ Aharoni-Simon M, Shumiatcher R, Yeung A, Shih AZ, Dolinsky VW, Doucette CA, et al. (June 2016). "Bcl-2 Regulates Reactive Oxygen Species Signaling and a Redox-Sensitive Mitochondrial Proton Leak in Mouse Pancreatic β-Cells". Endocrinology. 157 (6): 2270–2281. doi:10.1210/en.2015-1964. PMID 27070098.
- ^ García-Aranda M, Pérez-Ruiz E, Redondo M (December 2018). "Bcl-2 Inhibition to Overcome Resistance to Chemo- and Immunotherapy". International Journal of Molecular Sciences. 19 (12): 3950. doi:10.3390/ijms19123950. PMC 6321604. PMID 30544835.
- ^ Otake Y, Soundararajan S, Sengupta TK, Kio EA, Smith JC, Pineda-Roman M, et al. (April 2007). "Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA". Blood. 109 (7): 3069–3075. doi:10.1182/blood-2006-08-043257. PMC 1852223. PMID 17179226.
- ^ Vaux DL, Cory S, Adams JM (September 1988). "Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells". Nature. 335 (6189): 440–442. Bibcode:1988Natur.335..440V. doi:10.1038/335440a0. PMID 3262202. S2CID 23593952.
- ^ Kaiser U, Schilli M, Haag U, Neumann K, Kreipe H, Kogan E, et al. (August 1996). "Expression of bcl-2--protein in small cell lung cancer". Lung Cancer. 15 (1): 31–40. doi:10.1016/0169-5002(96)00568-5. PMID 8865121.
- ^ a b c Li A, Ojogho O, Escher A (2006). "Saving death: apoptosis for intervention in transplantation and autoimmunity". Clinical & Developmental Immunology. 13 (2–4): 273–282. doi:10.1080/17402520600834704. PMC 2270759. PMID 17162368.
- ^ a b Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF (January 2006). "Apoptotic mechanisms and the synaptic pathology of schizophrenia". Schizophrenia Research. 81 (1): 47–63. doi:10.1016/j.schres.2005.08.014. PMID 16226876. S2CID 22388783.
- ^ Chetty R, Cooper K, Gown AM, eds. (2016). "Section 1 - Antibodies: Bcl-2". Manual of Diagnostic Cytology (2nd ed.). Greenwich Medical Media, Ltd. pp. 23–24. doi:10.1017/9781139939508.013. ISBN 978-1-139-93950-8.
- ^ Dias N, Stein CA (November 2002). "Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides". European Journal of Pharmaceutics and Biopharmaceutics. 54 (3): 263–269. doi:10.1016/S0939-6411(02)00060-7. PMID 12445555.
- ^ Mavromatis BH, Cheson BD (June 2004). "Novel therapies for chronic lymphocytic leukemia". Blood Reviews. 18 (2): 137–148. doi:10.1016/S0268-960X(03)00039-0. PMID 15010151.
- ^ "Genasense (oblimersen sodium) FDA Approval Status". Drugs.com. Retrieved 11 February 2016.
- ^ Vogler M, Dinsdale D, Dyer MJ, Cohen GM (March 2009). "Bcl-2 inhibitors: small molecules with a big impact on cancer therapy". Cell Death and Differentiation. 16 (3): 360–367. doi:10.1038/cdd.2008.137. hdl:2381/4756. PMID 18806758. S2CID 24538054.
- ^ Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. (June 2005). "An inhibitor of Bcl-2 family proteins induces regression of solid tumours". Nature. 435 (7042): 677–681. Bibcode:2005Natur.435..677O. doi:10.1038/nature03579. PMID 15902208. S2CID 4335635.
- ^ Hann CL, Daniel VC, Sugar EA, Dobromilskaya I, Murphy SC, Cope L, et al. (April 2008). "Therapeutic efficacy of ABT-737, a selective inhibitor of BCL-2, in small cell lung cancer". Cancer Research. 68 (7): 2321–2328. doi:10.1158/0008-5472.can-07-5031. PMC 3159963. PMID 18381439.
- ^ Hauck P, Chao BH, Litz J, Krystal GW (April 2009). "Alterations in the Noxa/Mcl-1 axis determine sensitivity of small cell lung cancer to the BH3 mimetic ABT-737". Molecular Cancer Therapeutics. 8 (4): 883–892. doi:10.1158/1535-7163.MCT-08-1118. PMID 19372561. S2CID 19245418.
- ^ Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. (March 2011). "Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors". Journal of Clinical Oncology. 29 (7): 909–916. doi:10.1200/JCO.2010.31.6208. PMC 4668282. PMID 21282543.
- ^ Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. (June 2012). "Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer". Clinical Cancer Research. 18 (11): 3163–3169. doi:10.1158/1078-0432.CCR-11-3090. PMC 3715059. PMID 22496272.
- ^ Kaefer A, Yang J, Noertersheuser P, Mensing S, Humerickhouse R, Awni W, et al. (September 2014). "Mechanism-based pharmacokinetic/pharmacodynamic meta-analysis of navitoclax (ABT-263) induced thrombocytopenia". Cancer Chemotherapy and Pharmacology. 74 (3): 593–602. doi:10.1007/s00280-014-2530-9. PMID 25053389. S2CID 10685695.
- ^ Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. (March 2014). "Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia". Cancer Discovery. 4 (3): 362–375. doi:10.1158/2159-8290.CD-13-0609. PMC 3975047. PMID 24346116.
- ^ Liao G (12 August 2011). "ABT-199 BH-3 Mimetic Enters Phase Ia Trial For Chronic Lymphocytic Leukemia". Asian Scientist. Archived from the original on 18 July 2012. Retrieved 11 February 2016.
- ^ a b Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. (January 2016). "Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia". The New England Journal of Medicine. 374 (4): 311–322. doi:10.1056/NEJMoa1513257. PMC 7107002. PMID 26639348.
- ^ "'Miracle drug cured my cancer!': Amazing three-week recovery of Staffordshire sufferer". Stoke Sentinel. Archived from the original on 12 May 2014. Retrieved 10 May 2014.
- ^ Smith M (7 December 2015). "Hard-to-Treat CLL Yields to Investigational Drug".
- ^ a b Bankhead C (11 April 2016). "FDA Approves AbbVie's BCL-2 Targeting Drug for CLL". Medpage Today.
- ^ "FDA approves venetoclax for CLL or SLL, with or without 17p deletion, after one prior therapy". U.S. Food and Drug Administration. 24 March 2020.
- ^ Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, et al. (March 2019). "Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients with Progressive Chronic Lymphocytic Leukemia". Cancer Discovery. 9 (3): 342–353. doi:10.1158/2159-8290.CD-18-1119. PMID 30514704.
- ^ Liu J, Li S, Wang Q, Feng Y, Xing H, Yang X, et al. (May 2024). "Sonrotoclax overcomes BCL2 G101V mutation-induced venetoclax resistance in preclinical models of hematologic malignancy". Blood. 143 (18): 1825–1836. doi:10.1182/blood.2023019706. PMC 11076911. PMID 38211332.
- ^ a b c d Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, et al. (February 2004). "Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3". Cell. 116 (4): 527–540. doi:10.1016/s0092-8674(04)00162-x. PMID 14980220. S2CID 17808479.
- ^ Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, et al. (December 2001). "Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening". Journal of Medicinal Chemistry. 44 (25): 4313–4324. doi:10.1021/jm010016f. PMID 11728179.
- ^ Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, et al. (October 1997). "p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum". The Journal of Cell Biology. 139 (2): 327–338. doi:10.1083/jcb.139.2.327. PMC 2139787. PMID 9334338.
- ^ Zhang H, Nimmer P, Rosenberg SH, Ng SC, Joseph M (August 2002). "Development of a high-throughput fluorescence polarization assay for Bcl-x(L)". Analytical Biochemistry. 307 (1): 70–75. doi:10.1016/s0003-2697(02)00028-3. PMID 12137781.
- ^ a b c d e f Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, et al. (February 2005). "Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function". Molecular Cell. 17 (3): 393–403. doi:10.1016/j.molcel.2004.12.030. PMID 15694340.
- ^ O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, et al. (January 1998). "Bim: a novel member of the Bcl-2 family that promotes apoptosis". The EMBO Journal. 17 (2): 384–395. doi:10.1093/emboj/17.2.384. PMC 1170389. PMID 9430630.
- ^ Hsu SY, Lin P, Hsueh AJ (September 1998). "BOD (Bcl-2-related ovarian death gene) is an ovarian BH3 domain-containing proapoptotic Bcl-2 protein capable of dimerization with diverse antiapoptotic Bcl-2 members". Molecular Endocrinology. 12 (9): 1432–1440. doi:10.1210/mend.12.9.0166. PMID 9731710.
- ^ Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, et al. (November 1998). "Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein". Journal of Virology. 72 (11): 8586–8596. doi:10.1128/JVI.72.11.8586-8596.1998. PMC 110269. PMID 9765397.
- ^ Real PJ, Cao Y, Wang R, Nikolovska-Coleska Z, Sanz-Ortiz J, Wang S, et al. (November 2004). "Breast cancer cells can evade apoptosis-mediated selective killing by a novel small molecule inhibitor of Bcl-2". Cancer Research. 64 (21): 7947–7953. doi:10.1158/0008-5472.CAN-04-0945. PMID 15520201. S2CID 11807428.
- ^ Puthalakath H, Villunger A, O'Reilly LA, Beaumont JG, Coultas L, Cheney RE, et al. (September 2001). "Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis". Science. 293 (5536): 1829–1832. Bibcode:2001Sci...293.1829P. doi:10.1126/science.1062257. PMID 11546872. S2CID 5638023.
- ^ a b Qin W, Hu J, Guo M, Xu J, Li J, Yao G, et al. (August 2003). "BNIPL-2, a novel homologue of BNIP-2, interacts with Bcl-2 and Cdc42GAP in apoptosis". Biochemical and Biophysical Research Communications. 308 (2): 379–385. doi:10.1016/s0006-291x(03)01387-1. PMID 12901880.
- ^ a b Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, Elangovan B, et al. (October 1994). "Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins". Cell. 79 (2): 341–351. doi:10.1016/0092-8674(94)90202-X. PMID 7954800. S2CID 38609845.
- ^ Ray R, Chen G, Vande Velde C, Cizeau J, Park JH, Reed JC, et al. (January 2000). "BNIP3 heterodimerizes with Bcl-2/Bcl-X(L) and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites". The Journal of Biological Chemistry. 275 (2): 1439–1448. doi:10.1074/jbc.275.2.1439. PMID 10625696.
- ^ Yasuda M, Han JW, Dionne CA, Boyd JM, Chinnadurai G (February 1999). "BNIP3alpha: a human homolog of mitochondrial proapoptotic protein BNIP3". Cancer Research. 59 (3): 533–537. PMID 9973195.
- ^ Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ (January 1995). "Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death". Cell. 80 (2): 285–291. doi:10.1016/0092-8674(95)90411-5. PMID 7834748. S2CID 10343291.
- ^ a b Komatsu K, Miyashita T, Hang H, Hopkins KM, Zheng W, Cuddeback S, et al. (January 2000). "Human homologue of S. pombe Rad9 interacts with BCL-2/BCL-xL and promotes apoptosis". Nature Cell Biology. 2 (1): 1–6. doi:10.1038/71316. PMID 10620799. S2CID 52847351.
- ^ Hoetelmans RW (June 2004). "Nuclear partners of Bcl-2: Bax and PML". DNA and Cell Biology. 23 (6): 351–354. doi:10.1089/104454904323145236. PMID 15231068.
- ^ Oltvai ZN, Milliman CL, Korsmeyer SJ (August 1993). "Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death". Cell. 74 (4): 609–619. doi:10.1016/0092-8674(93)90509-O. PMID 8358790. S2CID 31151334.
- ^ Gillissen B, Essmann F, Graupner V, Stärck L, Radetzki S, Dörken B, et al. (July 2003). "Induction of cell death by the BH3-only Bcl-2 homolog Nbk/Bik is mediated by an entirely Bax-dependent mitochondrial pathway". The EMBO Journal. 22 (14): 3580–3590. doi:10.1093/emboj/cdg343. PMC 165613. PMID 12853473.
- ^ Wang HG, Rapp UR, Reed JC (November 1996). "Bcl-2 targets the protein kinase Raf-1 to mitochondria". Cell. 87 (4): 629–638. doi:10.1016/s0092-8674(00)81383-5. PMID 8929532. S2CID 16559750.
- ^ Gil-Parrado S, Fernández-Montalván A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, et al. (July 2002). "Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members". The Journal of Biological Chemistry. 277 (30): 27217–27226. doi:10.1074/jbc.M202945200. PMID 12000759.
- ^ Poulaki V, Mitsiades N, Romero ME, Tsokos M (June 2001). "Fas-mediated apoptosis in neuroblastoma requires mitochondrial activation and is inhibited by FLICE inhibitor protein and Bcl-2". Cancer Research. 61 (12): 4864–4872. PMID 11406564.
- ^ Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES (April 2002). "Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria". The Journal of Biological Chemistry. 277 (16): 13430–13437. doi:10.1074/jbc.M108029200. PMID 11832478.
- ^ Pathan N, Aime-Sempe C, Kitada S, Basu A, Haldar S, Reed JC (2001). "Microtubule-targeting drugs induce bcl-2 phosphorylation and association with Pin1". Neoplasia. 3 (6): 550–559. doi:10.1038/sj.neo.7900213. PMC 1506558. PMID 11774038.
- ^ Pathan N, Aime-Sempe C, Kitada S, Haldar S, Reed JC (2001). "Microtubule-targeting drugs induce Bcl-2 phosphorylation and association with Pin1". Neoplasia. 3 (1): 70–79. doi:10.1038/sj.neo.7900131. PMC 1505024. PMID 11326318.
- ^ Inohara N, Ding L, Chen S, Núñez G (April 1997). "harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L)". The EMBO Journal. 16 (7): 1686–1694. doi:10.1093/emboj/16.7.1686. PMC 1169772. PMID 9130713.
- ^ Ueno H, Kondo E, Yamamoto-Honda R, Tobe K, Nakamoto T, Sasaki K, et al. (February 2000). "Association of insulin receptor substrate proteins with Bcl-2 and their effects on its phosphorylation and antiapoptotic function". Molecular Biology of the Cell. 11 (2): 735–746. doi:10.1091/mbc.11.2.735. PMC 14806. PMID 10679027.
- ^ Jin Z, Gao F, Flagg T, Deng X (September 2004). "Tobacco-specific nitrosamine 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes functional cooperation of Bcl2 and c-Myc through phosphorylation in regulating cell survival and proliferation". The Journal of Biological Chemistry. 279 (38): 40209–40219. doi:10.1074/jbc.M404056200. PMID 15210690.
- ^ Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, et al. (May 2000). "Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis". Science. 288 (5468): 1053–1058. Bibcode:2000Sci...288.1053O. doi:10.1126/science.288.5468.1053. PMID 10807576.
- ^ Deng X, Ito T, Carr B, Mumby M, May WS (December 1998). "Reversible phosphorylation of Bcl2 following interleukin 3 or bryostatin 1 is mediated by direct interaction with protein phosphatase 2A". The Journal of Biological Chemistry. 273 (51): 34157–34163. doi:10.1074/jbc.273.51.34157. PMID 9852076.
- ^ Alberici A, Moratto D, Benussi L, Gasparini L, Ghidoni R, Gatta LB, et al. (October 1999). "Presenilin 1 protein directly interacts with Bcl-2". The Journal of Biological Chemistry. 274 (43): 30764–30769. doi:10.1074/jbc.274.43.30764. PMID 10521466.
- ^ Fernandez-Sarabia MJ, Bischoff JR (November 1993). "Bcl-2 associates with the ras-related protein R-ras p23". Nature. 366 (6452): 274–275. Bibcode:1993Natur.366..274F. doi:10.1038/366274a0. PMID 8232588. S2CID 4312803.
- ^ Tagami S, Eguchi Y, Kinoshita M, Takeda M, Tsujimoto Y (November 2000). "A novel protein, RTN-XS, interacts with both Bcl-XL and Bcl-2 on endoplasmic reticulum and reduces their anti-apoptotic activity". Oncogene. 19 (50): 5736–5746. doi:10.1038/sj.onc.1203948. PMID 11126360.
- ^ Iwahashi H, Eguchi Y, Yasuhara N, Hanafusa T, Matsuzawa Y, Tsujimoto Y (November 1997). "Synergistic anti-apoptotic activity between Bcl-2 and SMN implicated in spinal muscular atrophy". Nature. 390 (6658): 413–417. Bibcode:1997Natur.390..413I. doi:10.1038/37144. PMID 9389483. S2CID 1936633.
- ^ Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, et al. (July 2004). "Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria". Neuron. 43 (1): 19–30. doi:10.1016/j.neuron.2004.06.021. PMID 15233914. S2CID 18141051.
- ^ Naumovski L, Cleary ML (July 1996). "The p53-binding protein 53BP2 also interacts with Bc12 and impedes cell cycle progression at G2/M". Molecular and Cellular Biology. 16 (7): 3884–3892. doi:10.1128/MCB.16.7.3884. PMC 231385. PMID 8668206.
External links
[edit]- The Bcl-2 Family Database
- The Bcl-2 Family at celldeath.de
- bcl-2+Genes at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- c-bcl-2+Proteins at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Human BCL2 genome location and BCL2 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P10415 (Human Apoptosis regulator Bcl-2) at the PDBe-KB.