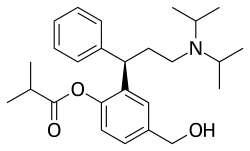
Fesoterodine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Fesobig , Toviaz |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a609021 |
| License data |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 52% (active metabolite) |
| Protein binding | 50% (active metabolite) |
| Metabolism | Liver (CYP2D6- and 3A4-mediated) |
| Elimination half-life | 7–8 hours (active metabolite) |
| Excretion | Kidney (70%) and fecal (7%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.184.854 |
| Chemical and physical data | |
| Formula | C26H37NO3 |
| Molar mass | 411.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Fesoterodine (INN, used as the fumarate under the brand name Toviaz) is an antimuscarinic drug developed by Schwarz Pharma AG to treat overactive bladder syndrome (OAB).[2] It was approved by the European Medicines Agency in April 2007,[3] the US Food and Drug Administration on October 31, 2008 [4] and Health Canada on February 9, 2012.[5]
Fesoterodine is a prodrug. It is broken down into its active metabolite, desfesoterodine, by plasma esterases.
Efficacy
[edit]Fesoterodine has the advantage of allowing more flexible dosage than other muscarinic antagonists.[6] Its tolerability and side effects are similar to other muscarinic antagonists and as a new drug seems unlikely to make great changes in practices of treatment for overactive bladder.[6]
A Japanese study from 2017, showed that urgency and urge incontinence are improved after 3 days administration of the drug, with full efficacy able to be judged after 7 days administration. Overactive bladder was found to be resolved in 88% of patients after seven days usage. [7]
References
[edit]- ^ "Product monograph brand safety updates". Health Canada. 6 June 2024. Retrieved 8 June 2024.
- ^ "Fesoterodine, New Drug Candidate For Treatment For Overactive Bladder – Pfizer To Acquire Exclusive Worldwide Rights". Medical News Today. 17 April 2006. Archived from the original on 16 May 2011. Retrieved 2 November 2007.
- ^ "Toviaz: European Public Assessment Report, Revision 3 - Published 02/06/08". European Medicines Agency. 2 June 2008. Archived from the original on 2008-04-01.
- ^ "Pfizer's Toviaz (fesoterodine fumarate) Receives FDA Approval for the Treatment of Overactive Bladder" (Press release). Pfizer Inc. 2008-10-31. Archived from the original on 2018-09-20. Retrieved 2008-11-06.
- ^ "Notice of Decision for TOVIAZ". Archived from the original on 2012-04-23. Retrieved 2012-04-20.
- ^ a b Vella M, Cardozo L (September 2011). "Review of fesoterodine". Expert Opinion on Drug Safety. 10 (5): 805–8. doi:10.1517/14740338.2011.591377. PMID 21639817. S2CID 9653506.
- ^ "Sato N, Fuji K, Ogawa Y (2017). "Transactions of The Showa University Society: The 335th Meeting". The Showa University Journal of Medical Sciences. 29 (2): 201–217. doi:10.15369/sujms.29.201. ISSN 2185-0968.
