Noma (disease)
| Noma | |
|---|---|
| Other names | cancrum oris, stomatitis gangrenosa |
 | |
| Stage 3 noma (gangrenous stage) in a young girl | |
| Specialty | Pediatrics, otorhinolaryngology, dentistry |
| Symptoms | Facial edema, fever, gangrene of face |
| Complications | sepsis & death, facial disfigurement, difficulty eating/drinking, social stigma |
| Usual onset | age 2-6 years |
| Duration | acute phase lasts 2-4 weeks |
| Causes | Opportunistic infection |
| Risk factors | Extreme poverty, malnutrition, immunosuppression |
| Diagnostic method | Based on symptoms |
| Differential diagnosis | Oral cancer, leishmaniasis, leprosy |
| Prevention | Adequate nutrition, oral hygiene |
| Treatment | Antibiotics, nutritional supplements, oral hygiene |
| Medication | antibiotics |
| Prognosis | 90% fatality rate without treatment |
| Frequency | 140,000 new cases per year (1998 estimate) |
Noma (also known as gangrenous stomatitis or cancrum oris) is a rapidly-progressive and often-fatal gangrenous infection of the mouth and face. Noma usually begins as an ulcer on gums and rapidly spreads into the jawbone, cheek, and soft tissues of the face. This is followed by death of the facial tissues and fatal sepsis. Survivors are left with severe facial disfigurement often with impairments in breathing, swallowing, speaking and vision.[1][2][3][4] In 2023 noma was added to the World Health Organization's list of neglected tropical diseases.[5]
This disease is strongly linked to poverty and malnutrition, and predominantly affects children between the ages of two and six years in the least developed countries around the world, primarily in sub-Saharan Africa; noma has also been seen in severely immunocompromised people in the developed world. It is preventable by proper nutrition and oral hygiene. Noma is most common in impoverished environments with poor healthcare infrastructure; as a result many cases go undiagnosed, untreated and unreported. There are no reliable estimates of its prevalence – in 1998 WHO estimated that there were 140 000 cases per year with a fatality rate of 90%; no more recent estimates are available.[1][2][3][4]
Noma is an opportunistic infection linked to a number of microbes which take advantage by malnutrition and compromised immunity. There is no evidence of direct transmission from person to person. In the early stages, it can be treated effectively with antibiotics and nutrition supplements. If diagnosed early enough, there can be proper wound-healing. After recovering, patients with disfigurement require complex surgical rehabilitation.[1][2][3][4]
Noma survivors experience high levels of stigma, social isolation, and discrimination within their communities. These can be countered by education and community outreach programs.[3]
Signs and symptoms
[edit]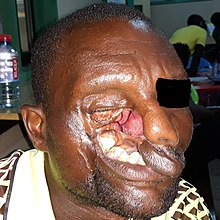
Initially, there may be a small ulcer in the mouth which progresses into necrotizing gingivitis - painful bleeding of the gums and inter-dental papillae. This is followed by a rapid spread of the infection resulting in more general inflammation of the mouth and lips, facial edema, and foul breath. If untreated, within a few days the necrotizing infection progresses into the facial muscles, the skin, and the upper and lower jaw resulting in tissue destruction and sloughing. Many patients die due to sepsis; survivors are left with permanent scarring and disfigurement.[6][7]
Noma neonatorum is a severe infection affecting very young or newborn children in impoverished environments. A gangrenous infection spreads across the oral, nasal and/or anal areas, and is frequently fatal. The pattern of lesions is similar to those found in noma.[8]
Stages of noma
[edit]The World Health Organization divides noma into five stages: Acute necrotizing gingivitis, edema, gangrenous, scarring, and sequelae.[9]
Warning-signs
[edit]Before the development of noma, there may be simple gingivitis: Inflammation and reddening of the gums, which bleed when touched or during brushing of the teeth. The WHO recommends disinfectant mouthwash; if not available, use warm, salted water that has been boiled. A high-protein diet, Vitamin A supplements, and patient education on oral hygiene are also recommended to prevent noma from progressing to the acute stages.[9]
Stage I: Acute necrotizing gingivitis
[edit]This is the first stage of noma. The gums are red or reddish-purple and bleed spontaneously. The child has fetid breath and may drool. Painful ulcers of the gums develop, causing trouble eating. If the patient is malnourished and has recently been sick with an infectious disease, such as measles or chickenpox, they are at more risk for developing noma. Fever may develop at this stage, which can persist indefinitely. Appropriate treatment at this stage can halt the disease.[9]
Stage II: Edema
[edit]This stage begins the acute phase of noma. The telltale sign is facial edema (swelling) of the lips, cheeks, eyes, etc. Ulceration of the gums worsens during this stage; ulceration may spread to the mucosa (soft, mucus-producing tissue) of the mouth and nose. The patient may feel pain or soreness in their mouth and cheeks. Other symptoms at this stage include fever, drooling, fetid breath, lymphadenopathy (swollen lymph nodes), and difficulty eating. Progression of the disease can be halted with appropriate treatment.[9]
Stage III: Gangrene
[edit]At this and subsequent stages, although the disease can still be treated, sequelae will inevitably set in. In this stage, the infection eats away at the soft tissue of the patient's face. The gangrene may affect the cheeks, lips, nose, mouth, and nasal and oral cavities. Dead tissue sloughs away over time, leaving holes in the face and the soft tissue, possibly exposing bones and teeth. The patient is apathetic, has little appetite, and has great difficulty eating.[9] At this stage, there is a high risk of sepsis leading to death.[7]
Stage IV: Scarring
[edit]The acute phase is over by this point, but the patient's life is still at risk and treatment is still recommended. This stage lasts one to two weeks. The patient may experience trismus (difficulty moving/opening the jaw), scars will form, and any exposed teeth will set in place.[9]
Stage V: Sequelae
[edit]The disease is over by this point, but sequelae from the gangrenous and scarring stages remain. Tissue may be missing, teeth may still be exposed, and the face is disfigured. The patient may have difficulty eating, drinking, and speaking. Teeth may become set in the wrong places, or be lost altogether. There may still be problems with drooling and with opening/closing the jaw. Reconstructive surgery is an option at this phase. Social reintegration is also very important.[9]

Epidemiology
[edit]As of December 2023[update], most people who acquire this disease are between the ages of two and six years old, living in the poorest countries of the world. Accurate figures for noma prevalence are not available due to difficulties in diagnosis and reporting in the endemic areas. In 1998 The World Health Organization estimated that 140,000 new cases were occurring each year, with a 90% fatality rate, and total of 770,000 surviving with scarring or disfigurement.[1]
Noma is associated with a very high morbidity,[10] and a mortality rate of approximately 90 percent. The prognosis is much better with treatment; if children have access to medical care, the mortality rate drops to under 10 percent.[11] After gangrene sets in, patients are likely to die of sepsis within one to two weeks.[12]
Causes and risks
[edit]Noma is an opportunistic rather than contagious infection.[11] No single pathogen has been associated with the disease (the causative organisms are common in many environments) and there are no documented cases of person to person transmission.[1][13] The underlying causes for this disease are extreme poverty, malnutrition, other causes of immunosuppression, underlying infections, and poor oral health.[1] The disease principally affects extremely impoverished and malnourished children between 2 and 6 years old in tropical regions. Cases of noma have also been reported in malnourished or immunosuppressed adults, and in concentration camps during the second world war.[14][12][15]
Predisposing factors include:[15][16][17]
- malnutrition and vitamin deficiency
- immunodeficiency
- poor oral hygiene
- recent illness (especially acute necrotizing ulcerative gingivitis, measles, malaria, or kwashiorkor),[9][18]
- social and environmental factors such as maternal malnutrition and closely-spaced pregnancies that result in offspring with weakened immune systems[9]
Treatment
[edit]When noma is detected at an early stage, its progression can be rapidly halted through basic hygiene, antibiotics and improved nutrition. However, its physical effects are permanent and may require oral and maxillofacial surgery or reconstructive plastic surgery to repair.[1] Treatments for noma in the acute stage include penicillin, sulfonamides,[12] and other antibiotics.
In all stages of noma, the World Health Organization encourages antibiotics, vitamin A supplements or other nutritional supplements, a high-protein diet, and proper hydration.
The World Health Organization recommends using amoxicillin and metronidazole in tandem to treat stage I noma (acute necrotizing gingivitis), along with the use of chlorhexidine and hydrogen peroxide to clean the mouth and gums.[9]
For stage II noma (edema phase), stage III noma (acute/gangrenous stage), and stage IV noma (scarring phase), the WHO recommends either one of two therapies. The first therapy includes the concurrent use of amoxicillin, clavulanic acid, gentamicin, and metronidazole. The second option includes the concurrent use of ampicillin, gentamicin, and metronidazole. For both options, chlorhexidine mouthwash is advised. For stage III and IV noma, the use of ketamine and honey are both given as options for dressing the lesions.[9]
Reconstruction is usually very challenging and should be delayed until full recovery (usually about one year following initial intervention).[19]
History
[edit]
Known in antiquity to such physicians as Hippocrates and Galen, noma was once reported around the world, including in Europe and the United States. The disease was well-known in the Netherlands in the 1500s and 1600s. The first clinical description of noma was in 1595 by a Dutch man, Carolus Battus. Dutch surgeon Cornelis van de Voorde first used the term "noma" to describe the disease in 1680. A European scientist, Gabriel Lund, attributed noma to poverty, cramped living conditions, and malnutrition in 1765. English physician John Addington Symmonds linked the disease to previous infection with measles. The first surgical treatment for noma sequelae was performed in 1781. Surgical treatments for sequelae developed throughout the 1800s. In the late 1800s, scientists suspected that noma was caused by bacteria.[12]
With improvements in hygiene and nutrition, noma has disappeared from industrialized countries since the 20th century, except during World War II when it was endemic to the Auschwitz and Belsen concentration camps.[14] The disease and treatments were studied by Berthold Epstein, a Czech physician and forced-labor prisoner who had recommended the study under Josef Mengele's direction.[14]
Since 1970, there has been little research done on noma, with few exceptions. One exception is Cyril Enwonwu, a Nigerian scientist focusing on noma.[12] Nigeria is also home to one of the few hospitals in the world that focuses on treating noma patients: Sokoto Noma Hospital, in the city of Sokoto.[20]
In January 2023 the Nigerian Ministry of Health submitted to the World Health Organization a request for noma to be added to WHO's list of neglected tropical diseases. This had been endorsed by 31 countries, and was accompanied by a dossier of evidence demonstrating that noma fit the criteria for inclusion. In December 2023 WHO conceded the request. It is hoped that this will encourage more research into the disease.[5][21]
Etymology
[edit]The word "noma" derives from the classical Greek word νομή, used to describe the continuing process of a fire or an ulcer.[22]
Society and culture
[edit]People with noma and noma survivors may face stigma. Some think that noma is a contagious disease, so they avoid noma sufferers and survivors to avoid contracting it.[23] Parents may hide afflicted children within the home because of social stigma, which can prevent them from getting treatment. Some also believe noma may be caused by witchcraft or a curse on the child's parents.[9] Based on one 1997 estimate, roughly 770,000 people worldwide live with noma sequelae. However, "noma is a disease of shame," and children are sometimes hidden in isolation rather than being sent to receive treatment.[11]
In Nigeria, sufferers and their families may seek traditional medicine rather than go to a medical center. In a study of 7,185 noma sufferers across Nigeria, only 19% reported going to a hospital or medical center upon discovering a facial lesion. 47.6% took 1–3 weeks to visit a hospital; the rest took longer to visit a hospital.[13]
Children and other noma survivors in Africa are helped by a few international charitable organizations, such as Facing Africa, a UK registered charity that helps affected Ethiopians, and Swiss charity Winds of Hope.[24] There is one dedicated noma hospital in Nigeria, the Noma Children Hospital Sokoto, staffed by resident and visiting medical teams supported by Médecins Sans Frontières. Some of the staff are noma survivors.[23][25] In other countries, such as Ethiopia, international charities work in collaboration with the local health care system to provide complex reconstructive surgery which can give back facial functions such as eating, speaking and smiling. Teams of volunteer medics coming from abroad are often needed to support the local capacity to address the most severe cases, which can be extremely challenging even for senior maxillofacial surgeons.[26] On 10 June 2010, the work of such volunteer surgeons was featured in a UK BBC Two documentary presented by Ben Fogle, Make Me a New Face: Hope for Africa's Hidden Children.[27][28]
See also
[edit]References
[edit]- ^ a b c d e f g "Noma – Key facts". World Health Organization. 15 December 2023. Retrieved 19 December 2023.
- ^ a b c Enwonwu, C.O.; Falkler, W.A.; Idigbe, E.O. (April 2000). "Oro-Facial Gangrene (Noma/Cancrum Oris): Pathogenetic Mechanisms". Critical Reviews in Oral Biology & Medicine. 11 (2): 159–171. doi:10.1177/10454411000110020201. ISSN 1045-4411.
- ^ a b c d "Issues & Crises – Noma". Médecins Sans Frontières (Doctors Without Borders). 4 January 2024. Retrieved 4 January 2024.
- ^ a b c Dutta, Sanchari Sinha (20 February 2023). "What is Noma (Cancrum oris)?". News-Medical.net. Retrieved 4 January 2024.
- ^ a b Johnson, Sarah (2023-12-15). "Survivors of disfiguring condition hail addition to WHO neglected diseases list". The Guardian. ISSN 0261-3077. Retrieved 2023-12-15.
- ^ MedlinePlus Medical Encyclopedia (28 April 2023). "Noma". National Institutes of Health MedlinePlus. Retrieved 19 December 2023.
- ^ a b Srour, M. Leila; Marck, Klaas; Baratti-Mayer, Denise (2017-02-08). "Noma: Overview of a Neglected Disease and Human Rights Violation". The American Journal of Tropical Medicine and Hygiene. 96 (2): 268–274. doi:10.4269/ajtmh.16-0718. ISSN 0002-9637. PMC 5303022. PMID 28093536.
- ^ T.B. Parikh; R.N. Nanavati; R.H. Udani. Noma Neonatorum Indian Journal of Pediatrics, Volume 73 – May, 2006
- ^ a b c d e f g h i j k l "Information brochure for early detection and management of noma" (PDF). World Health Organization: African Region. WHO Regional Office for Africa. 2016.
- ^ Barmes DE, Enwonwu CO, Leclercq MH, Bourgeois D, Falkler WA (1997). "The need for action against oro-facial gangrene (noma)". Trop Med Int Health. 2 (12): 1111–1114. doi:10.1046/j.1365-3156.1997.d01-220.x. PMID 9438464. S2CID 29871960.
- ^ a b c Tonna JE, Lewin MR, Mensh B (December 2010). Franco-Paredes C (ed.). "A case and review of noma". PLOS Neglected Tropical Diseases. 4 (12): e869. doi:10.1371/journal.pntd.0000869. PMC 3006140. PMID 21200428.
- ^ a b c d e Marck KW (April 2003). "A history of noma, the 'Face of Poverty'". Plastic and Reconstructive Surgery. 111 (5): 1702–1707. doi:10.1097/01.PRS.0000055445.84307.3C. PMID 12655218.
- ^ a b Ver-or, Ngutor; Iregbu, Chukwuemeka Kenneth; Taiwo, Olaniyi Olufemi; Afeleokhai, Ikhelua Thomas; Aza, Chiangi Gabriel; Adaji, Jeremiah Z.; Margima, Charles (2022). "Retrospective Characterization of Noma Cases Found Incidentally across Nigeria during Outreach Programs for Cleft Lip from 2011–2020". American Society of Tropical Medicine and Hygiene. 107 (5): 1132–1136. doi:10.4269/ajtmh.22-0388. PMC 9709002. PMID 36216317.
- ^ a b c Lifton RJ (1986). The Nazi Doctors: Medical Killing and Psychological Genocide. Basic Books. p. 361. ISBN 978-0-465-04905-9.
- ^ a b Enwonwu CO, Falkler WA, Phillips RS (July 8, 2006). "Noma (cancrum oris)". The Lancet. 368 (9530): 147–156. doi:10.1016/S0140-6736(06)69004-1. PMID 16829299. S2CID 10647321.
- ^ Auluck A, Pai KM (2005). "Noma: Life Cycle of a Devastating Sore - Case Report and Literature Review" (PDF). Journal of the Canadian Dental Association. 71 (10): 757–757c. PMID 16324228.
- ^ Enwonwu CO (2006). "Noma--the ulcer of extreme poverty". The New England Journal of Medicine. 354 (3): 221–224. doi:10.1056/NEJMp058193. PMID 16421362. S2CID 11654106.
- ^ Baratti-Mayer, Denise; Gayet-Ageron, Angèle; Hugonnet, Stéphane; François, Patrice; Pittet-Cuenod, Brigitte; Huyghe, Antoine; Bornand, Jacques-Etienne; Gervaix, Alain; Montandon, Denys; Schrenzel, Jacques; Mombelli, Andrea; Pittet, Didier (July 5, 2013). "Risk factors for noma disease: a 6-year, prospective, matched case-control study in Niger". Lancet Global Health. 1 (2): 87–96. doi:10.1016/S2214-109X(13)70015-9. ISSN 2214-109X. PMID 25104163.
- ^ Neville, Brad. Oral and Maxillofacial Pathology, 3rd ed., Saunders Book Company, 062008. 5.11.2
- ^ Barnhart, Max (April 1, 2023). "A deadly disease so neglected it's not even on the list of neglected tropical diseases". NPR.
- ^ "MSF joins noma survivors in celebrating inclusion in WHO neglected tropical diseases list". reliefweb.int. 15 December 2023. Retrieved 19 December 2023.
- ^ Marck, K.W. (September 2003). "Cancrum oris and noma: some etymological and historical remarks". British Journal of Plastic Surgery. 56 (6): 524–527. doi:10.1016/S0007-1226(03)00224-8. PMID 12946368.
- ^ a b Barnhart, Max (April 1, 2023). "A deadly disease so neglected it's not even on the list of neglected tropical diseases". NPR.
- ^ "Winds of Hope". www.windsofhope.org. Retrieved 2021-05-14.
- ^ "Noma – a neglected disease". Noma. Retrieved 2021-06-12.
- ^ Medical care Archived 2009-04-28 at the Wayback Machine at Project Harar
- ^ "Make Me a New Face: Hope for Africa's Hidden Children". BBC. June 2010. Retrieved January 13, 2016.
- ^ Fogle, Ben (July 6, 2010). "Ben's Documentary on Noma – BBC2". BenFogle.com. Archived from the original on April 11, 2018. Retrieved January 13, 2010.
Further reading
[edit]- Boss K, Marck K (2006). The Surgical Treatment of Noma (in Dutch). Alphen aan den Rijn : Belvédère/Mediadact. ISBN 978-90-71736-31-5.
